Dupixent® (dupilumab) Asthma
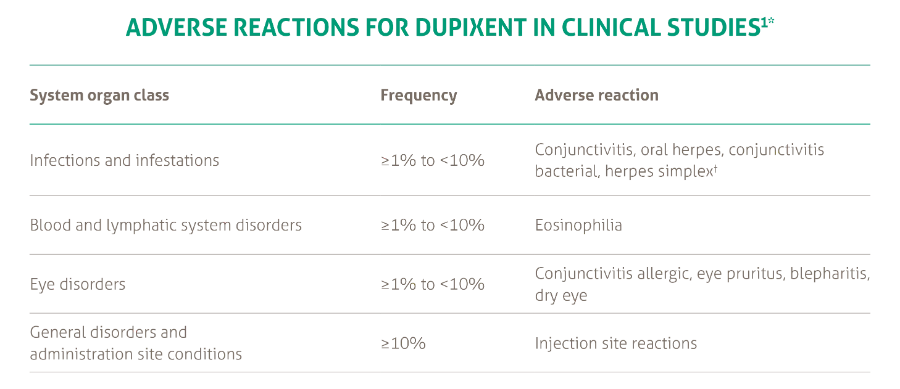
Dupixent is the first and only biologic with proven efficacy in severe asthma inhibiting IL-4 and IL-13 signalling.1 Dupixent is indicated as add on maintenance treatment in patients aged 6 years and older with moderate to severe asthma with type 2 inflammation (elevated eosinophils or elevated FeNO) that is inadequately controlled despite therapy with other medicinal products for maintenance treatment1
About Dupixent
For uncontrolled severe eosinophilic or allergic asthma
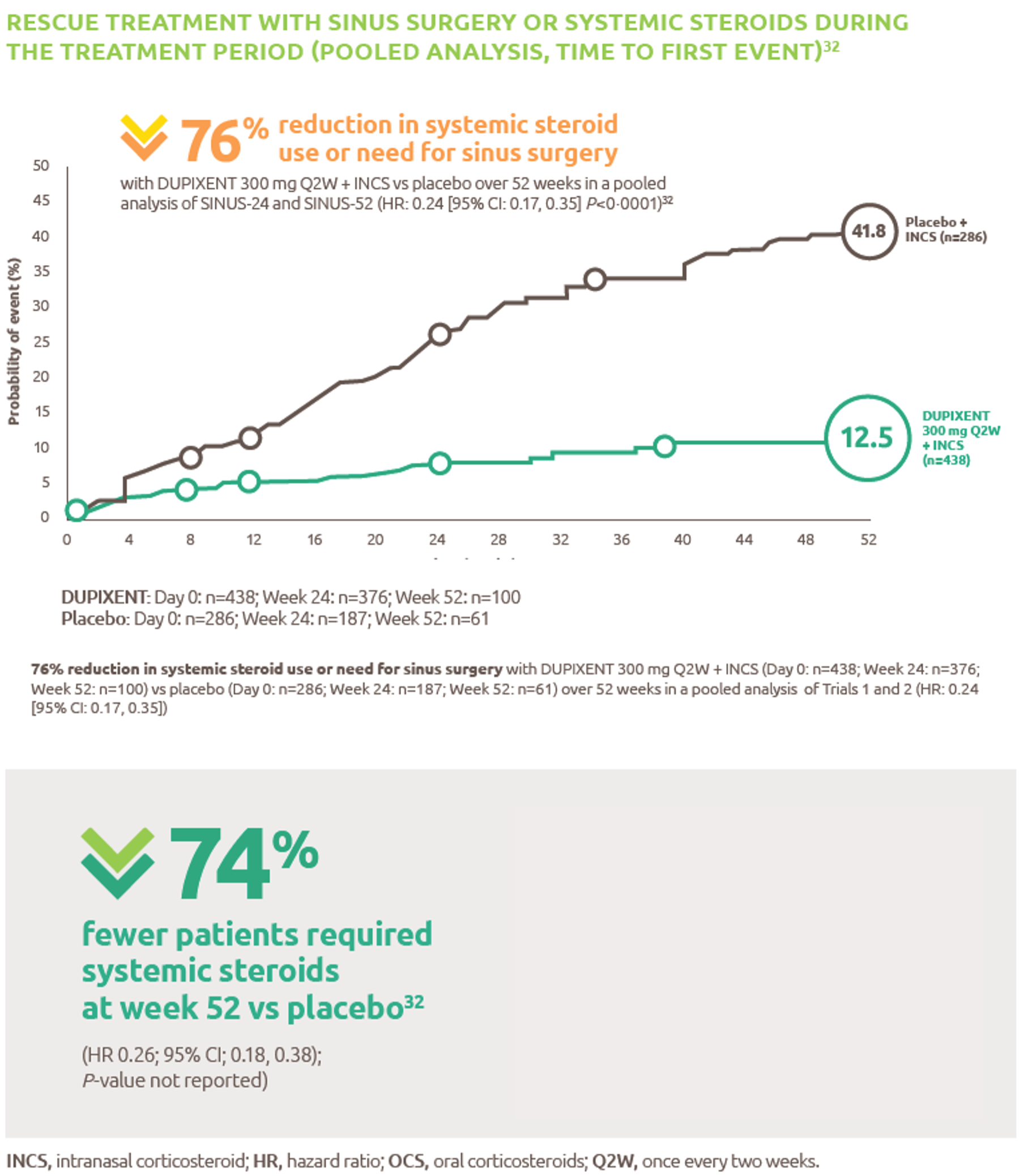
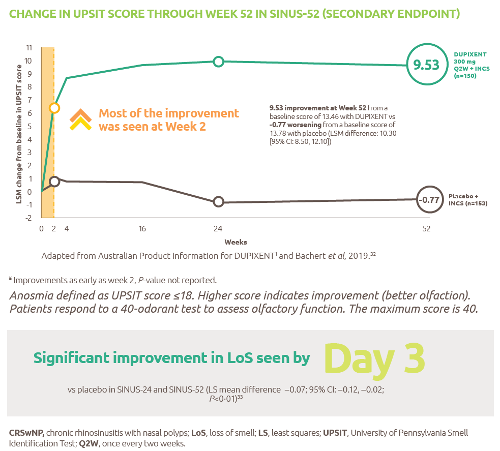
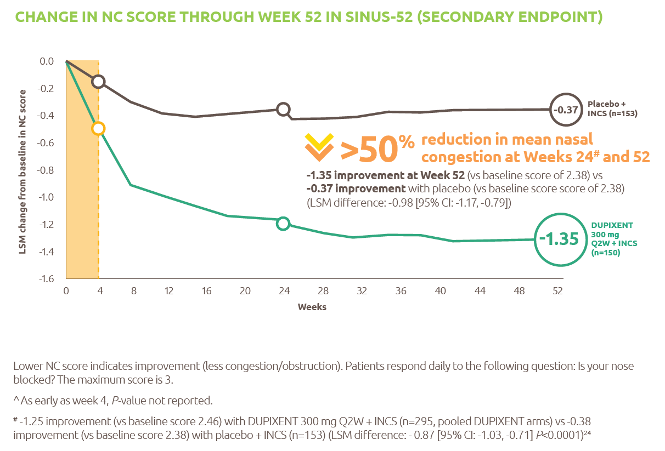
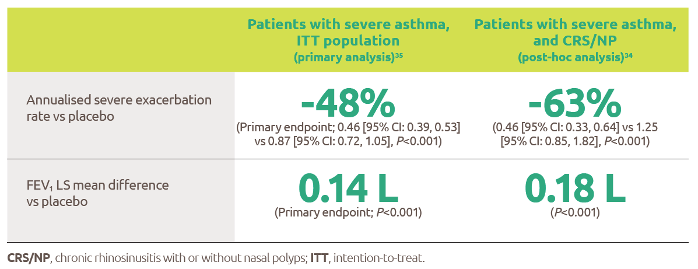
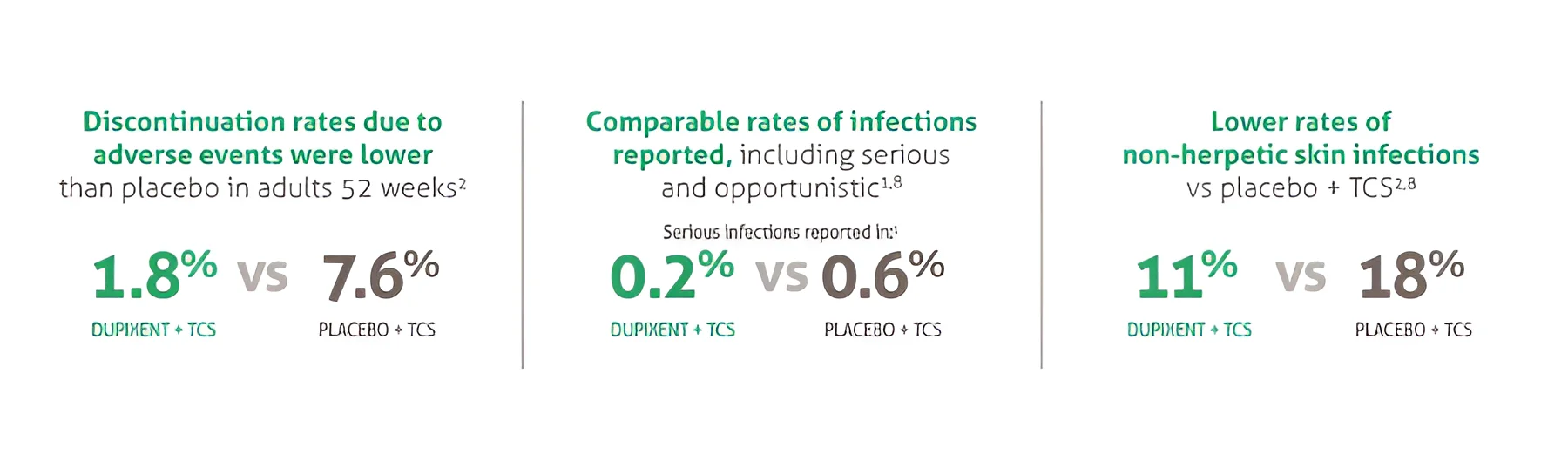
The only biologic indicated for patients with moderate to severe asthma with type 2 inflammation.1 Dupixent significantly reduced the annualised rate of severe exacerbations and improved FEV1 at Week 12 vs placebo (both P<0.001).2
For patients administered Dupixent as maintenance therapy for oral corticosteroid dependent asthma, Dupixent significantly reduced OCS use at Week 24 vs placebo (P<0.001).3
Don’t miss these key links

Need support?
We’re here to help.
For specific questions or support for your team, reach out to a Sanofi representative.
Indication and Patient Types
Clinical benefits have been established across patients with severe asthma1
"Difficulty breathing is a constant concern in my life ever since being diagnosed with asthma and later, nasal polyps. I worry about how asthma will affect me in the future."
"I want to play soccer with my grandkids, but my past asthma attacks frightened me and my family. Now I'm anxious about another attack every time they ask me to play outside. I don’t want to miss out on life’s precious moments because of this condition."
"I’m an animal lover, but I’m nervous about how my allergies might make my asthma worse. I was diagnosed with asthma when I was young, and I'm looking for a treatment that will allow me to volunteer at the animal shelter."
"It concerns me that I need multiple tablets every day on top of my regular inhaler to get my asthma under control. I have also heard about some of the potential side effects that you may get from corticosteroids and that sometimes makes me anxious."
*Hypothetical cases. Not actual patients.
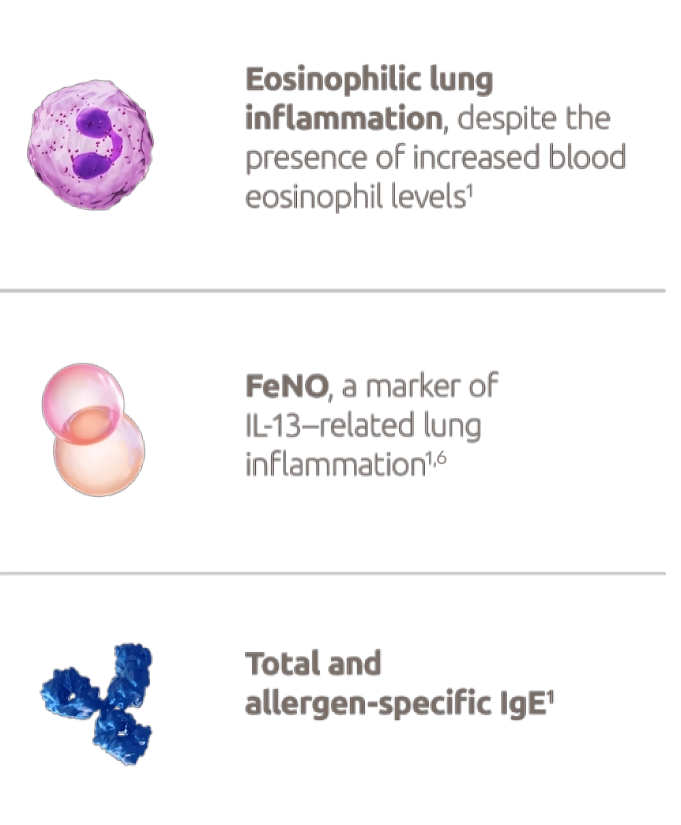
Type 2 inflammation could be signified by elevated eosinophilics or FeNO, or asthma that is clincally allergen-driven as defined by specific IgE or positive skin prick test for relevant allergens.1,4

Need support?
We’re here to help.
For specific questions or support for your team, reach out to a Sanofi representative.
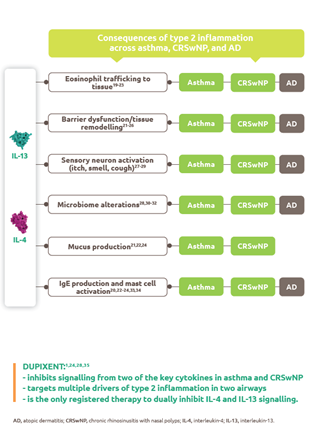
Mechanism of action
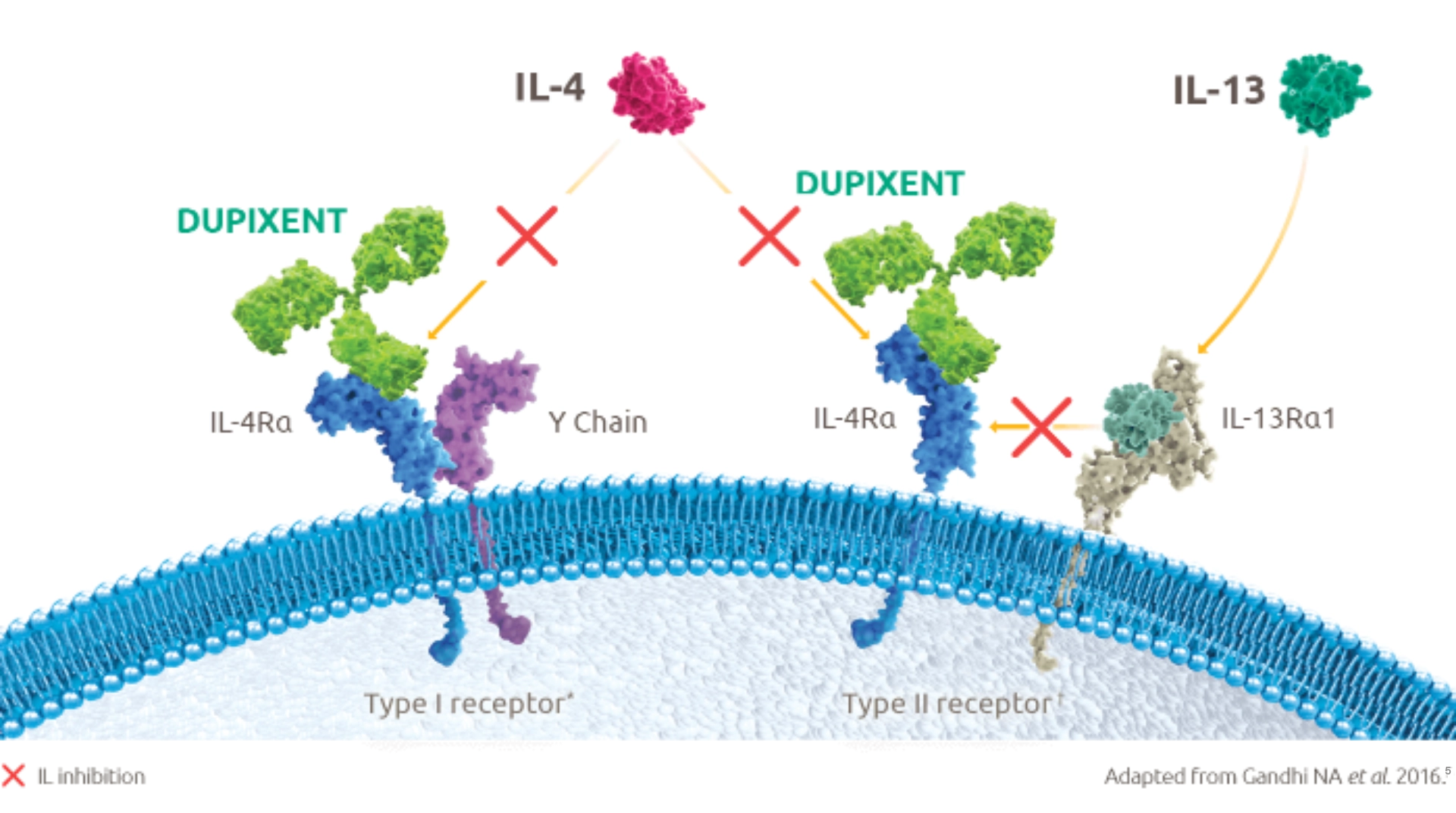
Dupixent is a human monoclonal antibody that specifically binds to the IL-4Rα subunit shared by receptor complexes for IL-4 and IL-131,4
No other registered biologic selectively inhibits signalling of two cytokines that drive type 2 inflammation5 to address multiple pathways in severe asthma and provide clinical benefits to a range of patients1
*The Type I receptor is found on B cells, T cells, monocytes, eosinophils, and fibroblasts.5 †The Type II receptor is found on epithelial cells, smooth muscle cells, fibroblasts, monocytes, and activated B cells.4
Dupixent blocks the downstream effects of IL-4 and IL-13, decreasing

Need support?
We’re here to help.
For specific questions or support for your team, reach out to a Sanofi representative.
FeNO: fractional exhaled nitric oxide. FEV1: forced expiratory volume in 1 second IL: interleukin. OCS: oral corticosteroid.
- Australian Approved Product Information for Dupixent (dupilumab).
- Castro M et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018; 378(26): 2486–96 (and supplementary appendix).
- Rabe KF et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018; 378(26): 2475–85 (and supplementary appendix).
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2022. Available at: www.ginasthma.org (accessed July 2022).
- Gandhi NA et al. Targeting key proximal drivers of Type 2 Inflammation in disease. Nat Rev Drug Discov 2016; 15(1): 35–50.
- Robinson D et al. Revisting Type 2-High and Type 2-Low airway Inflammation in asthma: current knwoledge and therapeutic implications. Clin Exp Allergy 2017; 47(2): 161–75.
Featured content
Evolving Asthma Landscape: Watch expert perspectives
Gain global clinical insights with international expert Prof. Nicola Hanania (USA) and respiratory physician Prof. Philip Bardin (Australia).
How to inject Dupixent (dupilumab) with pre-filled syringe
Step-by-step guide on how to administer Dupixent (200 and 300 mg) with a pre-filled syringe
Resources and support
Access a range of resources designed to support you and your patients throughout their treatment with Dupixent. From injection training videos to downloadable guides, these tools aim to enhance understanding and confidence in using Dupixent effectively.
Resources and tools
Patient support
Prescribing information
View product information document for full dosage information.
Can’t find what you’re looking for?
Search our extensive knowledge hub
PBS Information: Authority Required. Refer to PBS schedule for full authority Information.
Please review full Product Information before prescribing. Full Product Information is available here or by calling 1800 818 806.
▼This medicinal product is subject to additional monitoring in Australia. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse events at www.tga.gov.au/reporting-problems.
CRSwNP: chronic rhinosinusitis with nasal polyposis. FeNO: fractional exhaled nitric oxide. FEV1: forced expiratory volume in 1 second. ICS: inhaled corticosteroid. IgE: immunoglobulin E. IL: interleukin. LABA: long-acting beta agonist. LTRA: leukotriene receptor antagonist. OCS: oral corticosteroid.
- Australian Approved Product Information for Dupixent (dupilumab).
- Castro M et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018; 378(26): 2486–96 (and supplementary appendix).
- Rabe KF et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018; 378(26): 2475–85 (and supplementary appendix).
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2022. Available at: www.ginasthma.org (accessed July 2022).
- Gandhi NA et al. Targeting key proximal drivers of Type 2 Inflammation in disease. Nat Rev Drug Discov 2016; 15(1): 35–50.
- Robinson D et al. Revisting Type 2-High and Type 2-Low airway Inflammation in asthma: current knwoledge and therapeutic implications. Clin Exp Allergy 2017; 47(2): 161–75.
MAT-AU-2300926 - 8.0 - 11/2025


-Asthma.png/jcr:content.png)


%20self-injection%20training%20video%20-%20CB.png)
-Dosing-Guide---thumbnail.png/jcr:content.png)
.png/jcr:content.jpg)

-injection-guide.png/jcr:content.png)
.png/jcr:content.png)
.png/jcr:content.png)















.webp/jcr:content/RESP-ICT2-Wark_400X300%20(1).webp)
.webp/jcr:content/RESP-ICT2-Stone_400X300%20(1).webp)
.webp/jcr:content/RESP-ICT2-Tellus_400X300%20(1).webp)


.png)