Pain and GI symptoms management
PERIPHERAL NERVOUS SYSTEM INVOLVEMENT IN CLASSIC FABRY DISEASE: NEUROPATHIC PAIN AND GI SYMPTOMS1-7
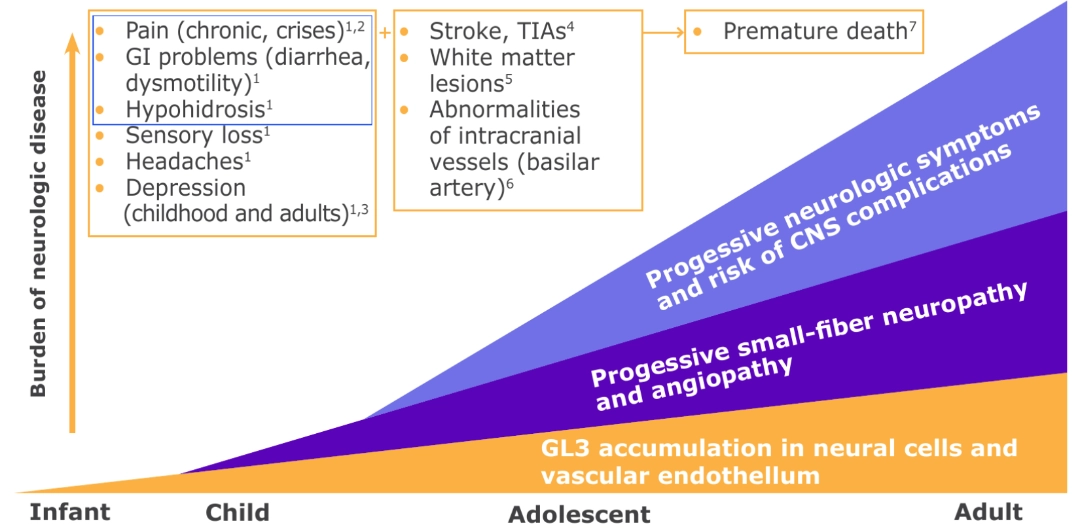
- Neuropathic pain and GI manifestations are frequent and often are the earliest symptoms of Fabry disease.
- Understanding the underlying pathophysiology is essential during patient assessment, to guide appropriate management strategies.
Note, when you press "Watch the webinar!" you are leaving our website and entering an external site - The Rare Diseases University. To access the content, you will need to create an account.
FOUR DISTINCT PAIN PHENOTYPES2
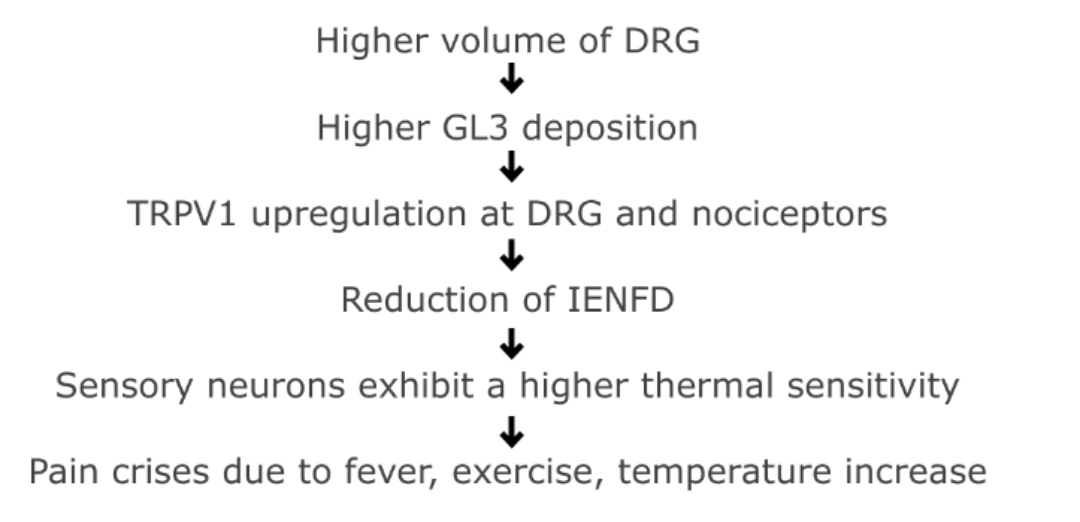
PATHOPHYSIOLOGY OF PAIN9-14
Lyso-GL3 enhances voltage-gated calcium currents in sensory neurons and causes pain.
GI MANIFESTATIONS15
◊ Abdominal cramps
◊ Vomiting
◊ Diarrhea
◊ Diverticular perforation
◊ Pseudo-obstruction
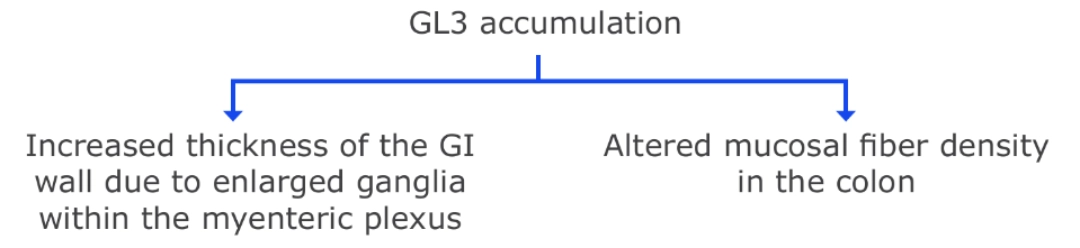
PATHOPHYSIOLOGY OF GI INVOLVEMENT15,16
Some patients with Fabry disease have demonstrated severe delay in gastric emptying.
WHEN TO START TREATMENT?21
• Symptomatic patients of any age or sex, including children with mild symptoms, should receive Fabry disease-specific and symptomatic treatment to manage symptoms and
slow disease progression.
• For asymptomatic boys with classical Fabry mutations, clinicians should consider
starting ERT around ages 8–10.
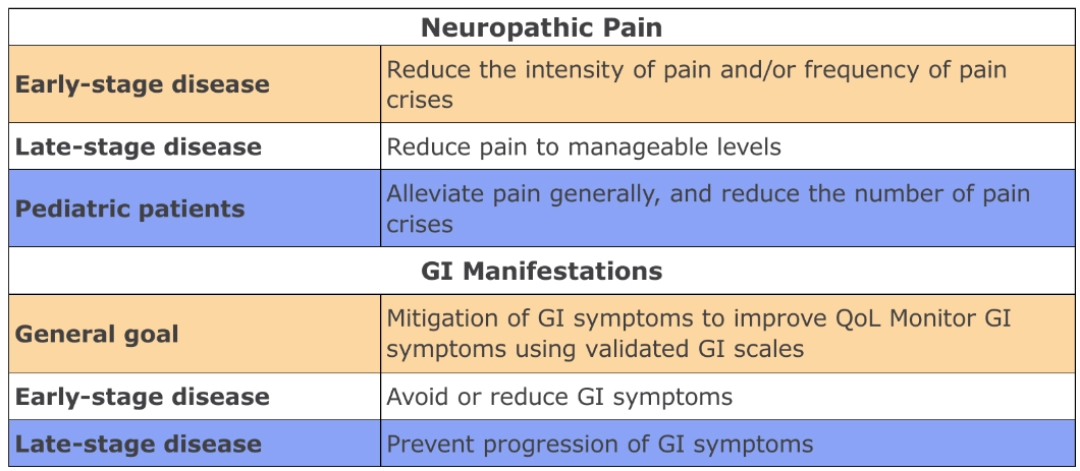
THERAPEUTIC GOALS22
“The general therapeutic goal for optimizing patient management in Fabry disease, should be to optimize both disease-specific and nonspecific adjunctive treatments to prevent/minimize effects of organ damage (e.g., kidney dysfunction) and prevent clinical events (e.g., stroke) as well as control symptoms, such as neuropathic pain.”
Wanner C, et al. Mol Genet Metab. 2018;124:189-203.
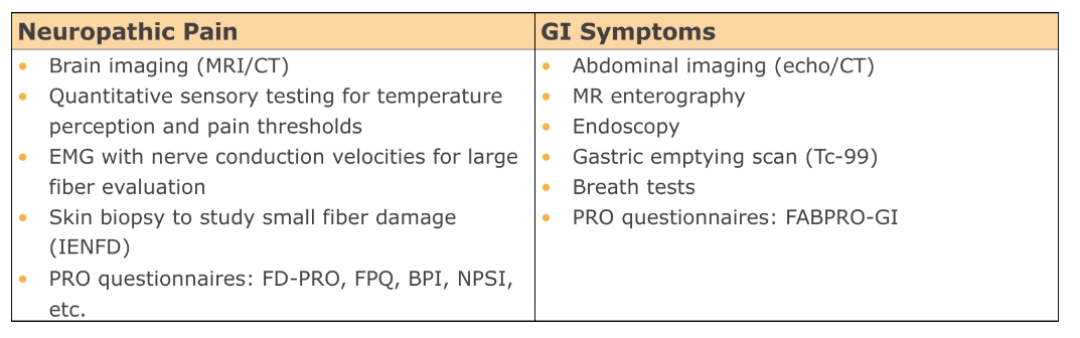
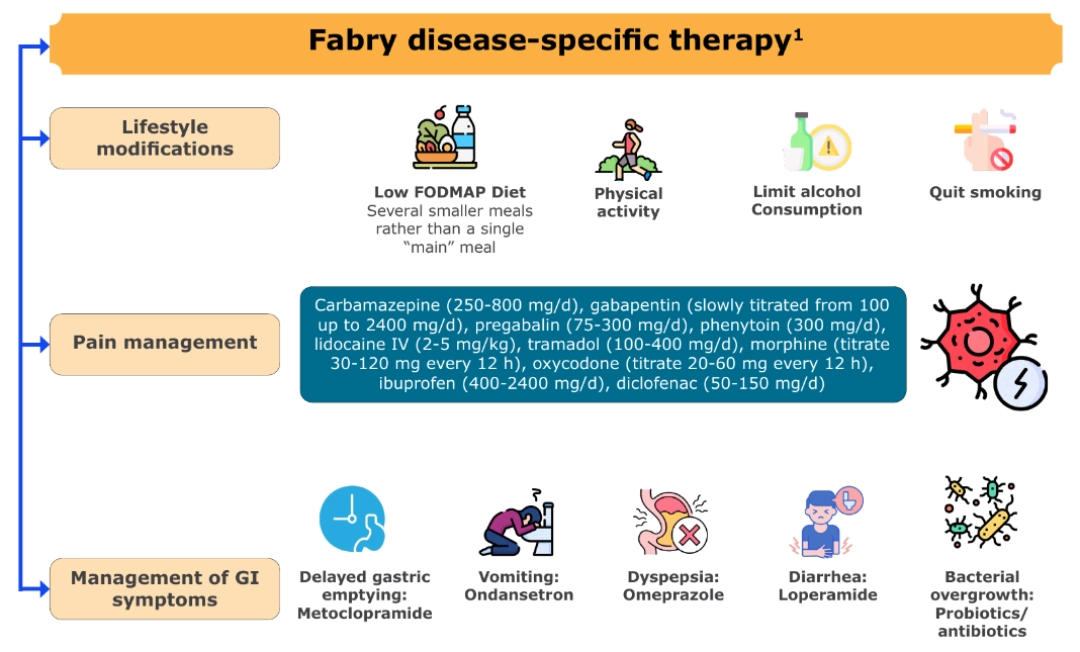
MULTIDIMENSIONAL MANAGEMENT STRATEGY15,23-25
treatment at an early stage to prevent disease progression.
o Delayed diagnosis can erode patient trust in the healthcare system, leading to
reduced adherence to prescribed therapies.
o Stigma around psychiatric conditions and misconceptions about mental health
services, may further complicate care delivery.
o Educating the patient about realistic expectations for management of these
symptoms and implementing personalized follow-up plans is critical to long-term
success.
BPI, Brief Pain Inventory; CNS, central nervous system; CT, computed tomography; d, day; DRG, dorsal root ganglion; echo, echocardiogram; ERT, enzyme replacement therapy; EMG, electromyography; FABPRO-GI, FABry Disease Patient-Reported Outcome-GastroIntestinal; FD-PRO, Fabry Disease Patient-Reported Outcome; FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols; FPQ, Fabry Pain Questionnaire; GI, gastrointestinal; GL3, globotriaosylceramide; IENFD, intraepidermal nerve fiber density; IV, intravenous; MR, magnetic resonance; MRI, magnetic resonance imaging; NPSI, Neuropathic Pain Symptom Inventory; PRO, patient-reported outcome; QoL, quality of life; Tc-99, technetium-99; TIA, transient ischemic attack; TRPV1, transient receptor potential vanilloid 1.
1. Ries M, et al. Eur J Pediatr. 2003;162:767-72.
2. Üçeyler N, et al. Clin J Pain. 2014;30:915-20.
3. Cole AL, et al. J Inherit Metab Dis. 2007;30:943-51.
4. Sims K, et al. Stroke. 2009;40:788-94.
5. Moore DF, et al. Brain Res Bull. 2003;62:231-40.
6. Fellgiebel A, et al. Neurology. 2009;72:63-8.
7. Waldek S, et al. Genet Med. 2009;11:790-96.
8. Desnick RJ, et al. In: Valle DL, et al. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill Education; 2019. 9. Gayathri N, et al. Ann Indian Acad Neurol. 2008;11:182-4.
10. deVeber GA, et al. Ann Neurol. 1992;31:409-15.
11. Kahn P. J Neurol Neurosurg Psychiatry. 1973;36:1053-62.
12. Lakomá J, et al. Mol Pain. 2016;12:1744806916663729.
13. Liguori R, et al. Muscle Nerve. 2010;41:409-12.
14. Choi L, et al. Neurosci Lett. 2015;594:163-8.
15. Politei JM, et al. Rare Dis Orphan Drugs J. 2024;3:17.
16. Masotti M, et al. Neurogastroenterol Motil. 2019;31:e13529.
17. Burlina AP, et al. BMC Neurol. 2011;11:61.
18. Hamed A, et al. Mol Genet Metab Rep. 2021;29:100824.
19. Jovanovic A, et al. Orphanet J Rare Dis. 2020;15:296.
20. Shields AL, et al. Qual Life Res. 2021;30:2983-2994.
21. Hopkin RJ, et al. Mol Genet Metab. 2016;117:104-13.
22. Wanner C, et al. Mol Genet Metab. 2018;124:189-203.
23. Ortiz A, et al. Mol Genet Metab. 2018;123:416-427.
24. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of CKD. Kidney Int. 2024;105:S117–S314.
25. Politei JM, et al. CNS Neurosci Ther. 2016;22:568-76.