Indication1
DUPIXENT is indicated as an add-on therapy with intranasal corticosteroids for the treatment of adults with severe CRSwNP for whom therapy with systemic corticosteroids and/or surgery do not provide adequate disease control.
CRS, chronic rhinosinusitis; CRSwNP, chronic rhinosinusitis with nasal polyps.
| TARGET Type 2 inflammation1-3 |
TREAT full spectrum of symptoms1,12,13 |
CONTROL with less surgery or fewer steroids1,13 |
| Find out how > | See the data > | Learn more > |
NP & Type 2 inflammation
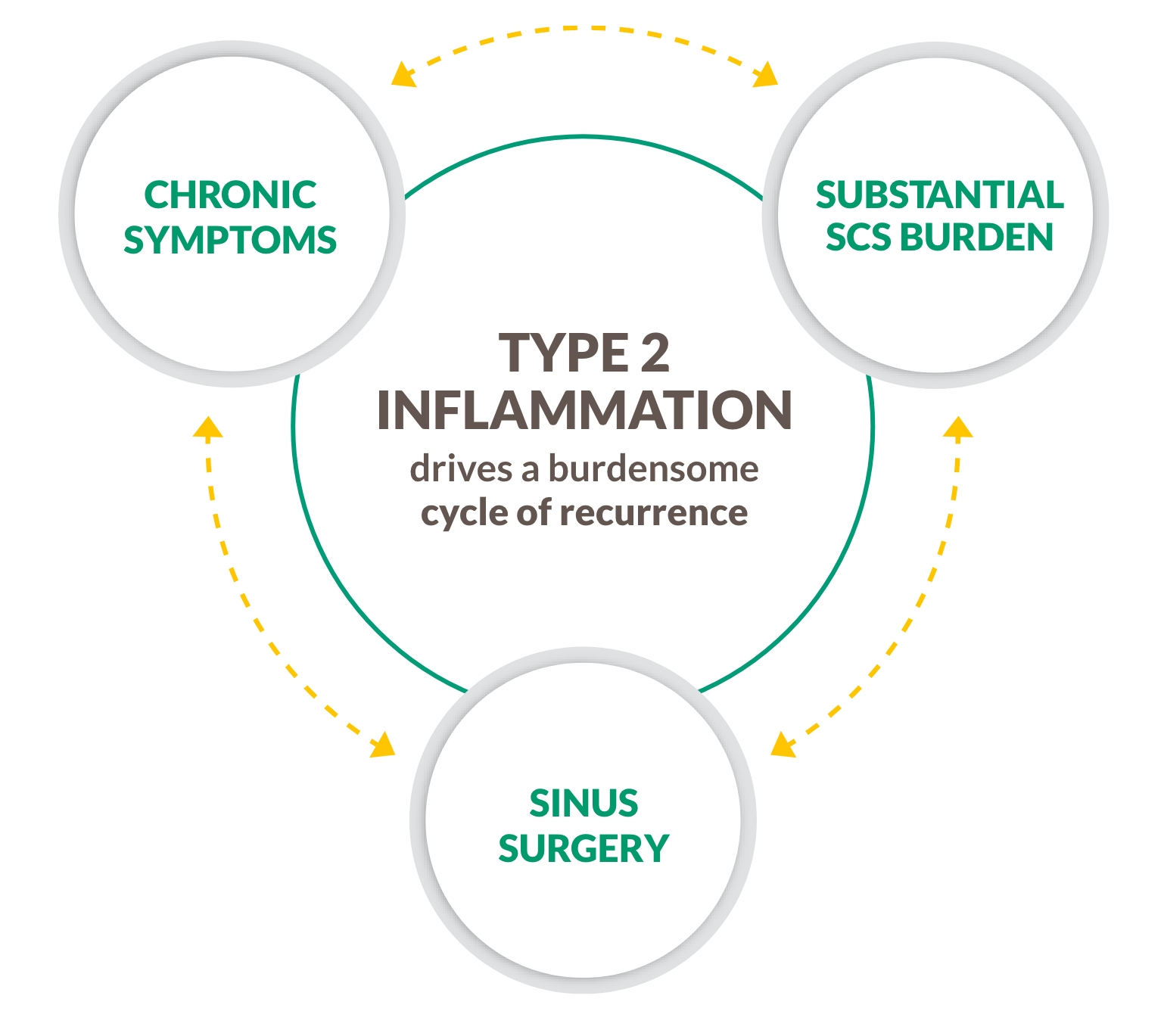
Unmet need–Cycle of Recurrence
CRS with nasal polyps is not just an obstructive disease
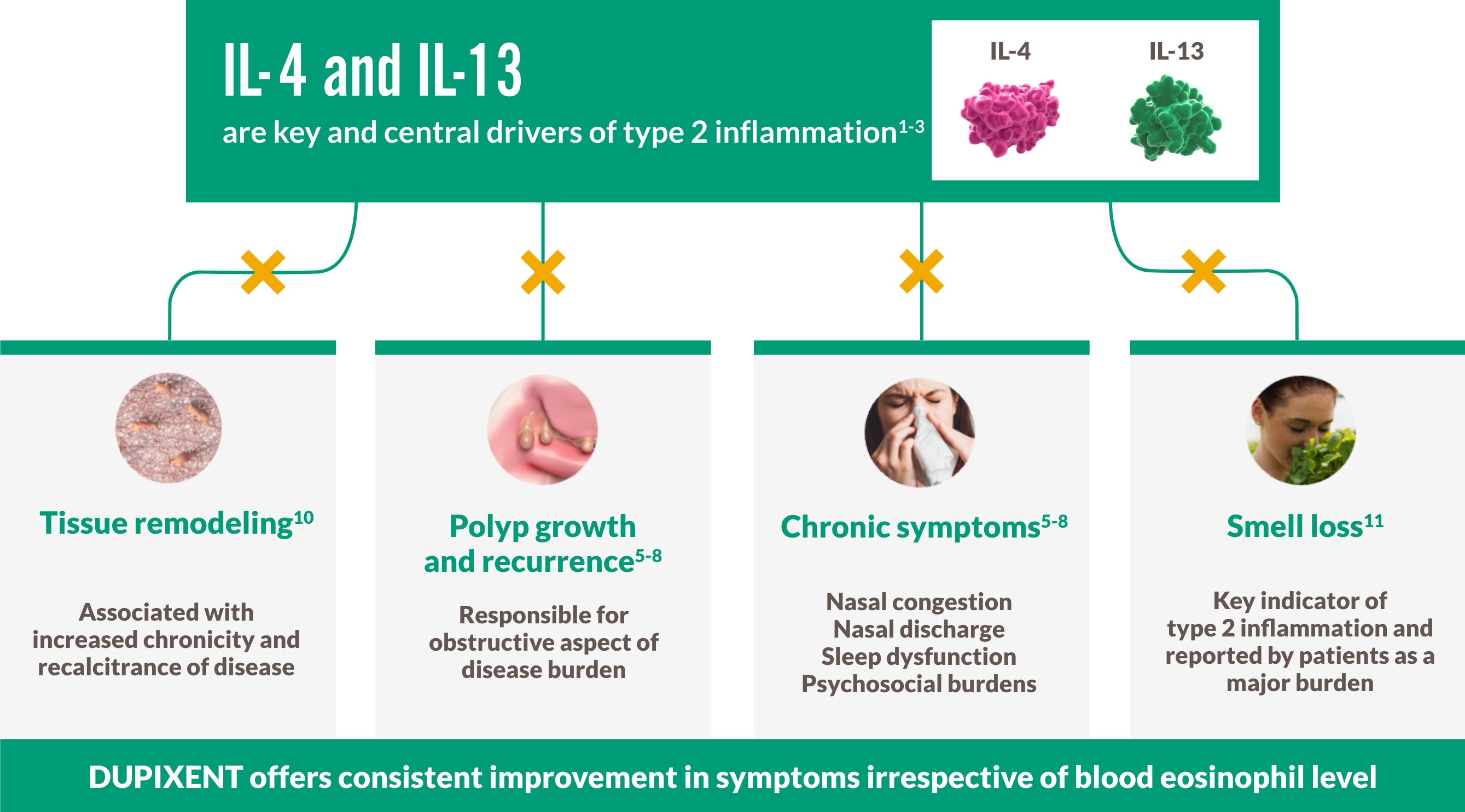
CRS WITH NASAL POLYPS IS A CHRONIC TYPE 2 INFLAMMATORY DISEASE4-9
~9 out of 10 patients with CRS with nasal polyps have Type 2 inflammation, an underlying driver of complications including polyp formation4-9

BREAK THE CYCLE WITH A BIOLOGIC THAT TARGETS TYPE 2 INFLAMMATION4-9
SCS, systemic corticosteroids.
MOA graphic
DUPIXENT CENTRALLY TARGETS UNDERLYING TYPE 2 INFLAMMATION IN CRS WITH NASAL POLYPS1-3
Efficacy and Burden data
Smell
PATIENTS REGAINED SMELL AS EARLY AS DAY 312,a
DUPIXENT improved smell loss (LoS) as early as Day 3, with a significantly greater proportion of patients with sense of smell (UPSIT score >18) vs placebo at Week 24 and sustained through Week 52 in patients receiving INCS1,12-14
Change in UPSIT score through Week 52 in SINUS-52 (secondary endpoint)13
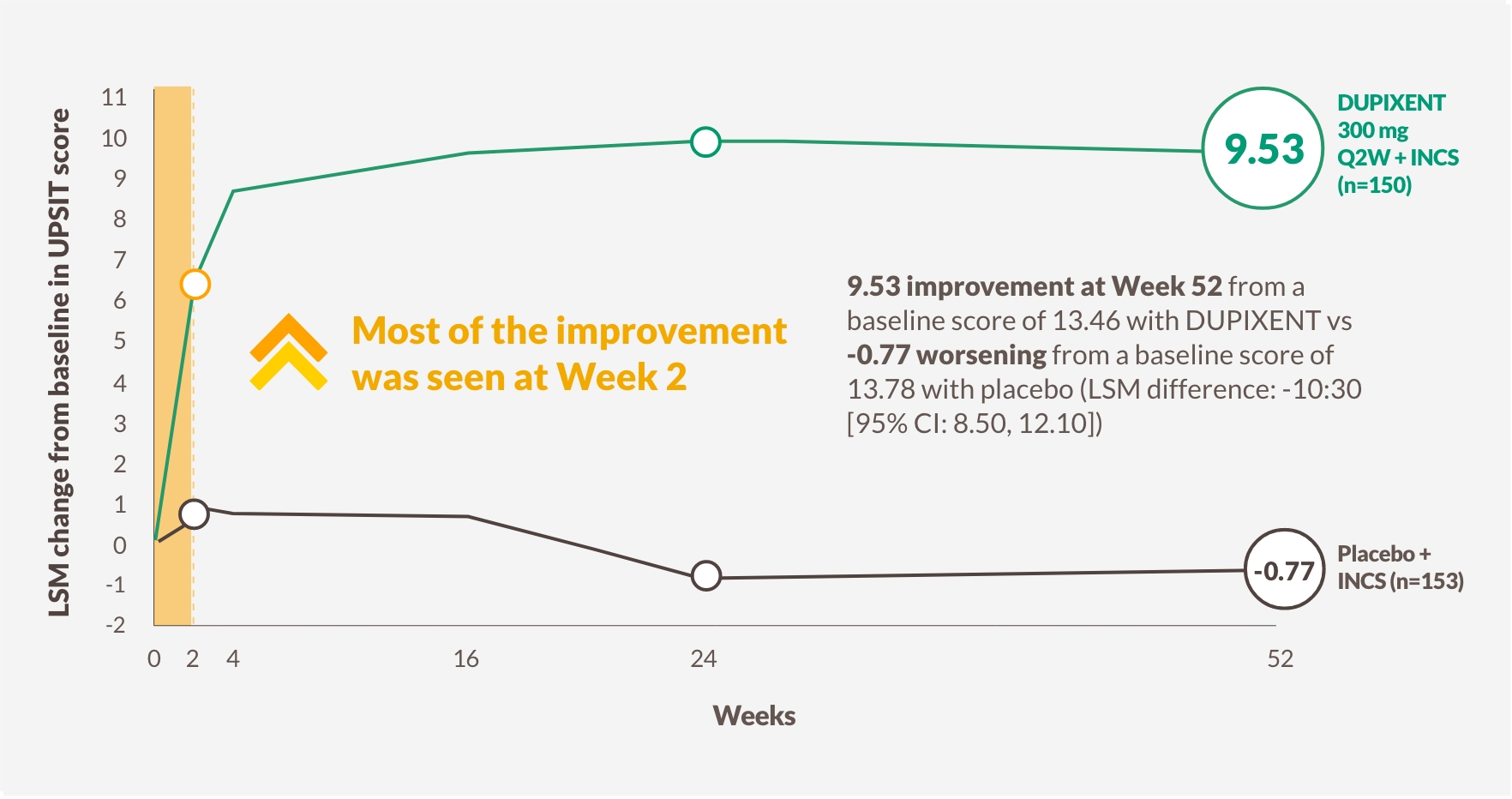
The figure is reproduced by Sanofi based on table S3C.13
a LSM difference between DUPIXENT (n=438) and placebo (n=286): -0.07 (95% CI: -0.12, -0.02).
b 79% (n=228/287) of patents in the pooled arm taking DUPIXENT 300 mg Q2W + INCS had anosmia at baseline, which was reduced to 30% (n=84/280) as per UPSIT score at Week 24.
Anosmia, UPSIT score ≤18; INCS, intranasal corticosteroids; LoS, loss of smell score is a patient-collected assessment of the daily symptom severity of decreased or loss of smell; LSM, least squares mean; Q2W, once every 2 weeks; UPSIT, University of Pennsylvania Smell Identification Test.

2 OUT OF 3 PATIENTS WERE ABLE TO SMELL AGAIN AT WEEK 521,13,b
SMELL LOSS IS ONE OF THE MOST IMPORTANT SYMPTOMS FOR PATIENTS15-18
~9 out of 10 patients who have CRS with nasal polyps experience loss of smell, which is correlated with increased disease severity and may be the first sign in the cycle of disease recurrence15-18
Smell loss is strongly associated with type 2 inflammation15-18
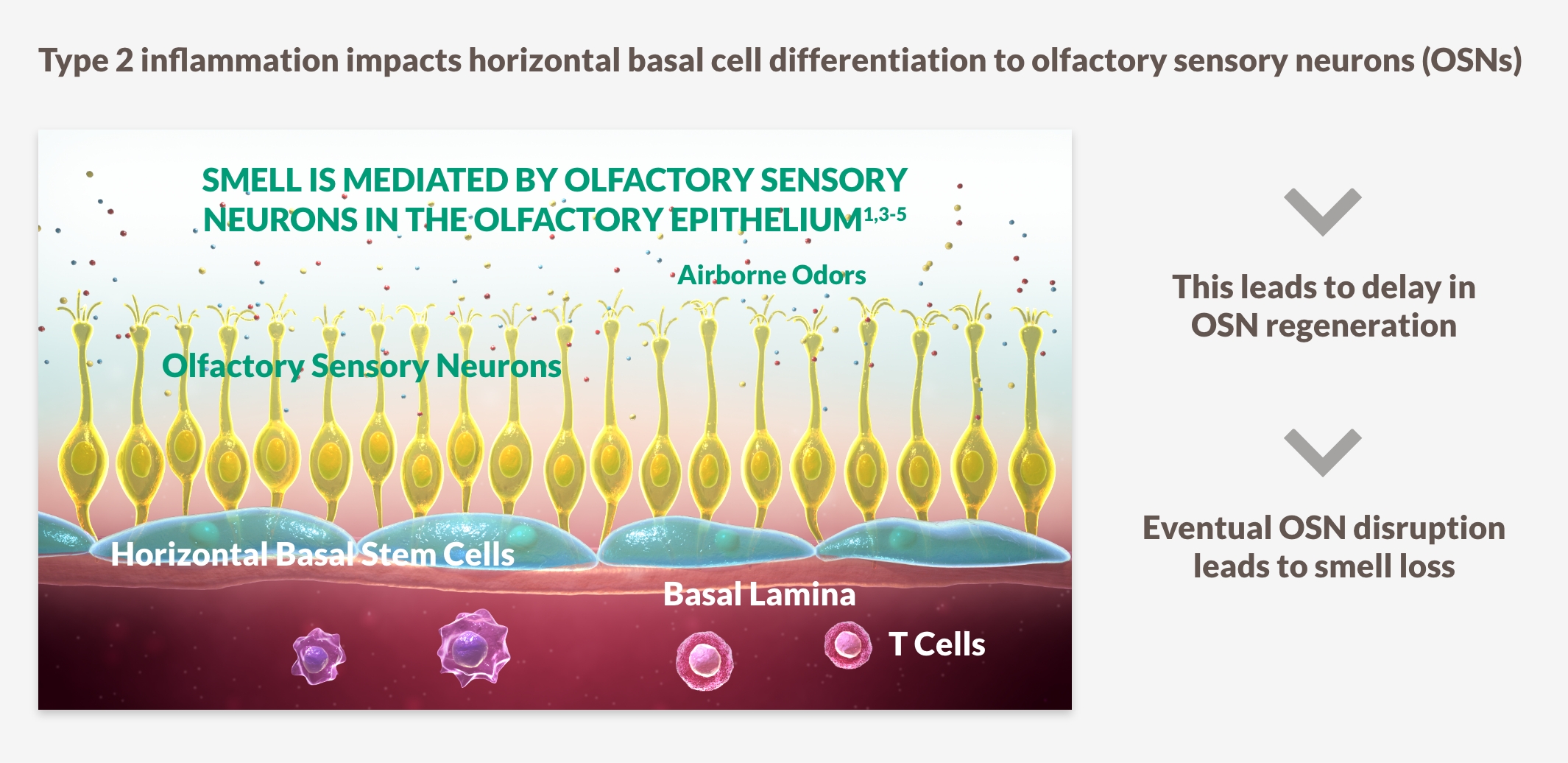

INHIBITING DRIVERS OF TYPE 2 INFLAMMATION MAY PLAY A ROLE IN IMPROVING SENSE OF SMELL15-18
Nasal congestion
RAPID IMPROVEMENT IN NASAL CONGESTION AT DAY 2 SUSTAINED OVER 52 WEEKS1,12,13
DUPIXENT improved NC score vs placebo as early as Day 2, with significant results at Week 24 and sustained through Week 52 in patients receiving INCS1,12,13
Change in NC score through Week 52 in SINUS-52 (secondary endpoint)13
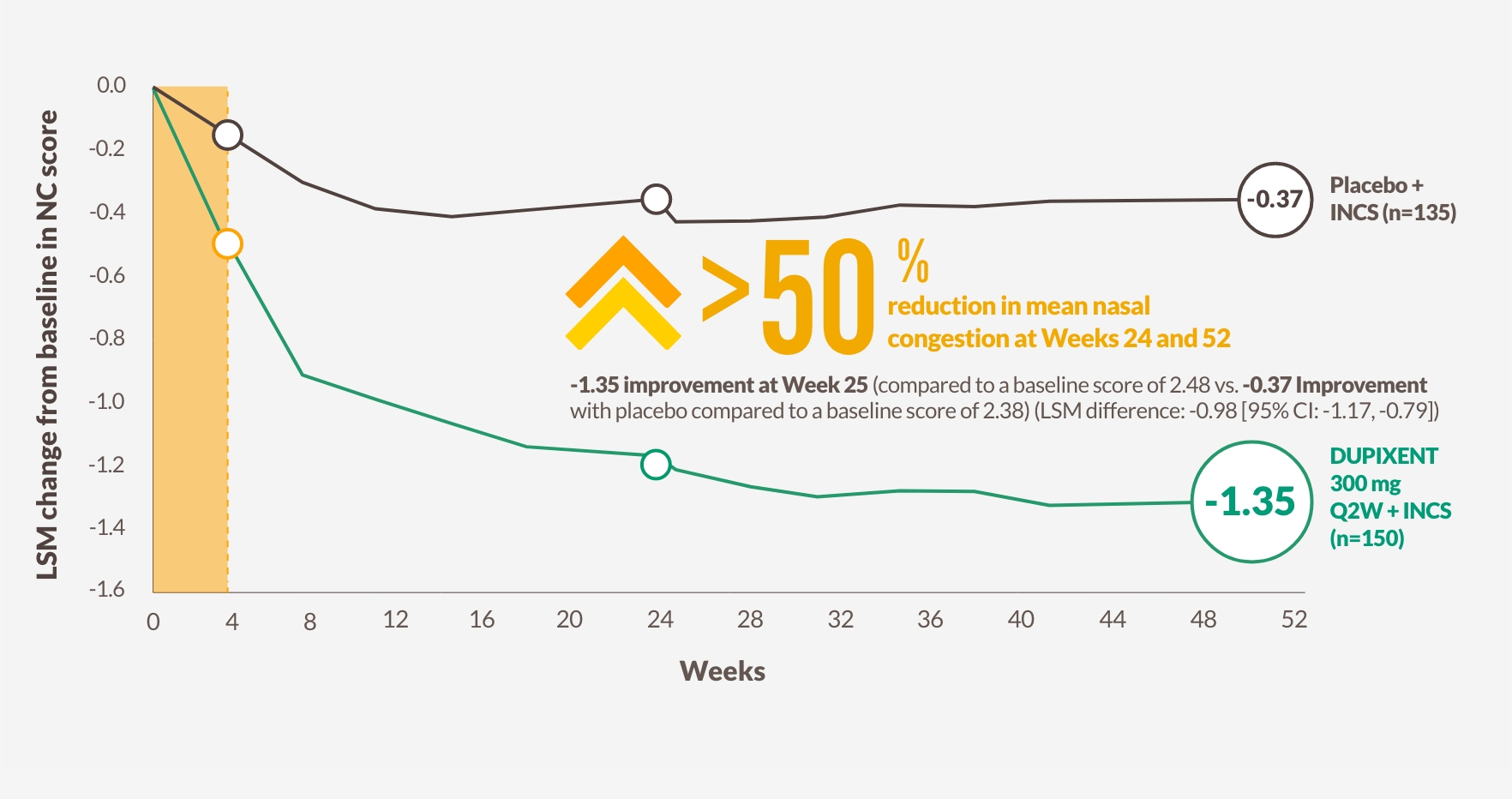
The figure is reproduced by Sanofi based on figure 2B13
-1.25 improvement at Week 24 (co-primary endpoint) from a baseline score of 2.46 with DUPIXENT 300 mg Q2W + INCS (n=295, pooled DUPIXENT arms) vs -0.38 improvement from a baseline score of 2.38 with placebo + INCS (n=153) (LSM difference: -0.87 [95% CI: -1.03, -0.71])1
HRQoL, health-related quality of life; NC, nasal congestion/obstruction.
UNDERLYING TYPE 2 INFLAMMATION CAN DRIVE CONGESTION AND REDUCE QUALITY OF LIFE19
Nasal congestion drives many physical, social, and psychological HRQoL burdens related to CRS with nasal polyps19
Impacts of nasal congestion on HRQoL19 |
|
|
|

MANY PATIENTS REPORTED FRUSTRATION WITH THE MANAGEMENT OF THEIR SYMPTOMS19
Surgery Reduction
LESS SURGERY FOR A MAJORITY OF PATIENTS1,13
DUPIXENT significantly reduced the need for sinus surgery vs placebo in over 52 weeks in patients receiving INCS1,13

83% reduction in the need for initial or repeat sinus surgery1,13
From a prespecified pooled analysis (dupilumab 300 mg Q2W [Arm A] and 300 mg Q2W–Q4W [Arm B] pooled; First 24 weeks only for Arm B).2
†100 mg MFNS in each nostril twice daily.2
‡Saline nasal lavage, systemic antibiotics, short-course SCS, or sinonasal surgery were permitted as needed during the treatment and follow-up periods.13 Pooled placebo n=286, Pooled dupilumab n=438.
SURGERY MAY STILL LEAVE PATIENTS IN A CYCLE OF RECURRENCE20-22
Despite addressing polyp obstruction, surgery does not address the underlying Type 2 inflammation, which is associated with the disease burden of CRS with nasal polyps, including new polyp formation20-22
Burdens and limitations of surgery20-22 |
||
|
With each sinus surgery: |
||
|
Risk of revision surgeries increases and duration between surgery decreases20 |
Sinus surgery does not address smell loss21 |
35% polyp recurrence rate within 6 months, increasing over time22 |

EPOS GUIDELINES CONSIDER A REDUCED NEED FOR SURGERY AS A CRITERION OF BIOLOGIC RESPONSE22
EPOS, European Position Paper on Rhinosinusitis and Nasal Polyps 2020.
Steroid Reduction
DUPIXENT TARGETS TYPE 2 INFLAMMATION TO REDUCE THE NEED FOR SYSTEMIC STEROIDS1,13
DUPIXENT significantly reduced the need for systemic steroids vs placebo over 52 weeks in patients receiving INCS1,13

74% reduction in the need for systemic corticosteroid use1,13
From a prespecified pooled analysis (dupilumab 300 mg Q2W [Arm A] and 300 mg Q2W–Q4W [Arm B] pooled; first 24 weeks only for Arm B).2
†100 mg MFNS in each nostril twice daily.2
‡Saline nasal lavage, systemic antibiotics, short-course SCS, or sinonasal surgery were permitted as needed during the treatment and follow-up periods.13
Pooled placebo n=286, Pooled dupilumab n=438.

DUPIXENT REDUCES OR ELIMINATES STEROID BURDEN REGARDLESS OF PRIOR SURGERY1
CUMULATIVE STEROID USE ADDS TO THE BURDENSOME CYCLE PATIENTS FACE22-24
SCS treatment broadly targets inflammation, and even short-term use carries a risk of serious short- and long-term side effects22-24
Limitations of SCS use22-24 |
||
|
The EPOS 2020 criteria advise not to prescribe more than two courses of SCS per year because of the cumulative side-effects. |
Risk of SCS use22-24 |
||
|

EPOS GUIDELINES CONSIDER A REDUCED NEED FOR SCS AS A CRITERION OF BIOLOGIC RESPONSE22
NP Score
THE ONLY APPROVED BIOLOGIC TO REPORT A >2-POINT NPS IMPROVEMENT IN PHASE 3 TRIALS1,25,26
DUPIXENT significantly reduced the size of nasal polyps vs placebo as measured by bilateral endoscopic NPS as early as Week 4, sustained through Week 521,25,26
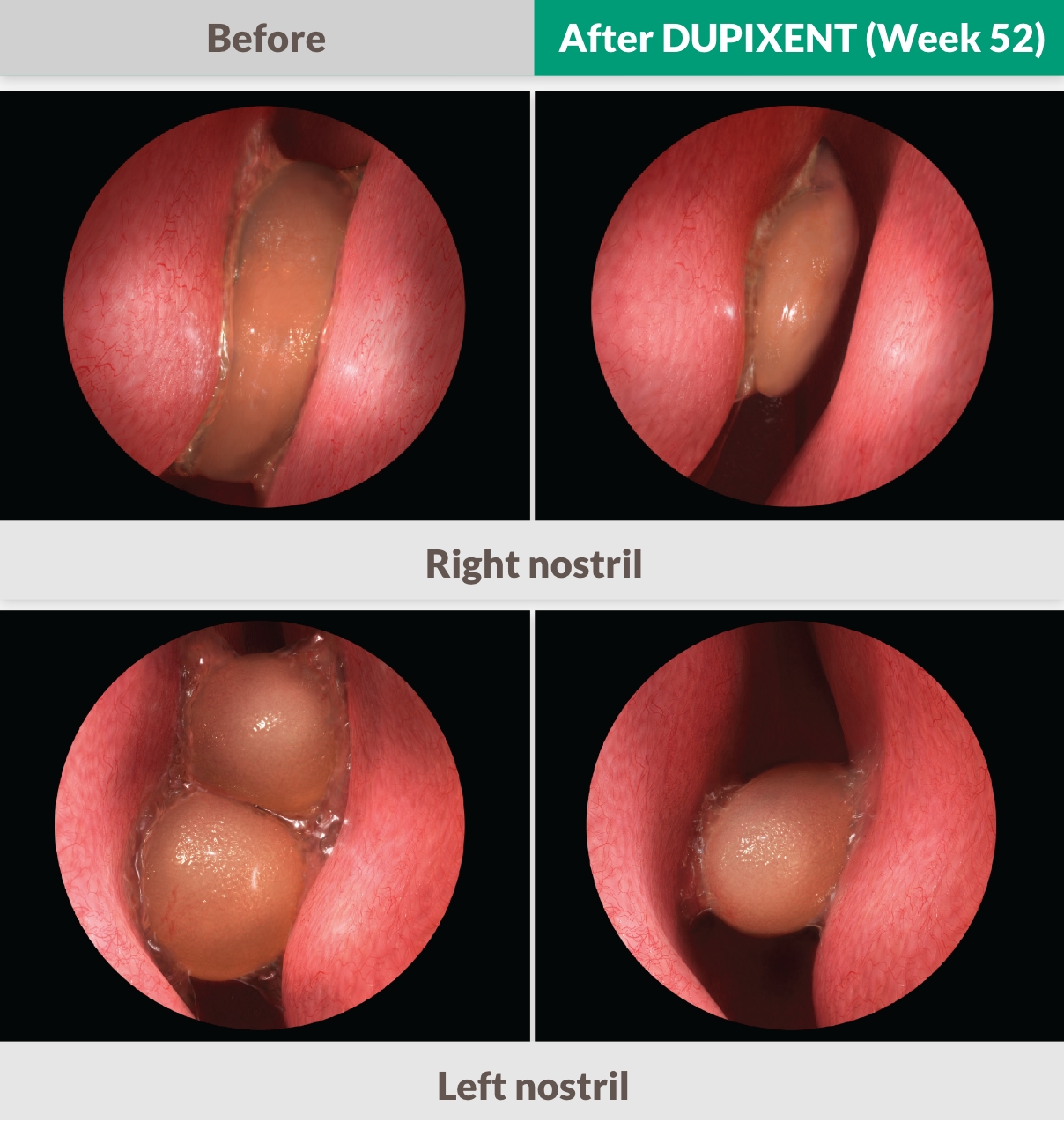
Endoscopy images adapted from actual SINUS-52 patient images. Individual results did vary.

37% improvement at Week 521,13
-1.71 improvement at Week 24 (co-primary endpoint) from a baseline score of 6.18 with DUPIXENT 300 mg Q2W + INCS (n=295, pooled DUPIXENT arms) vs 0.10 worsening from a baseline score of 5.96 with placebo + INCS (n=153) (LSM difference: -1.80 [95% CI: -2.10, -1.51])1
-2.24 improvement at week 52 from a baseline score of 6.07 (secondary endpoint) with DUPIXENT 300 mg Q2W + INCS (n=150) vs 3% worsening with placebo + INCS (n=153) (0.15 from a baseline score of 5.96) (LSM difference: -2.40 [95% CI: -2.77, -2.02])1
NASAL POLYPS ARE A MANIFESTATION OF CRS—TYPE 2 INFLAMMATION IS AN UNDERLYING CAUSE11
Bilateral endoscopic NPS can help measure polyp formation and recurrence, which are key signs of Type 2 inflammation11,27,28
NPS improvement >2 indicates substantial relief of obstruction27,28
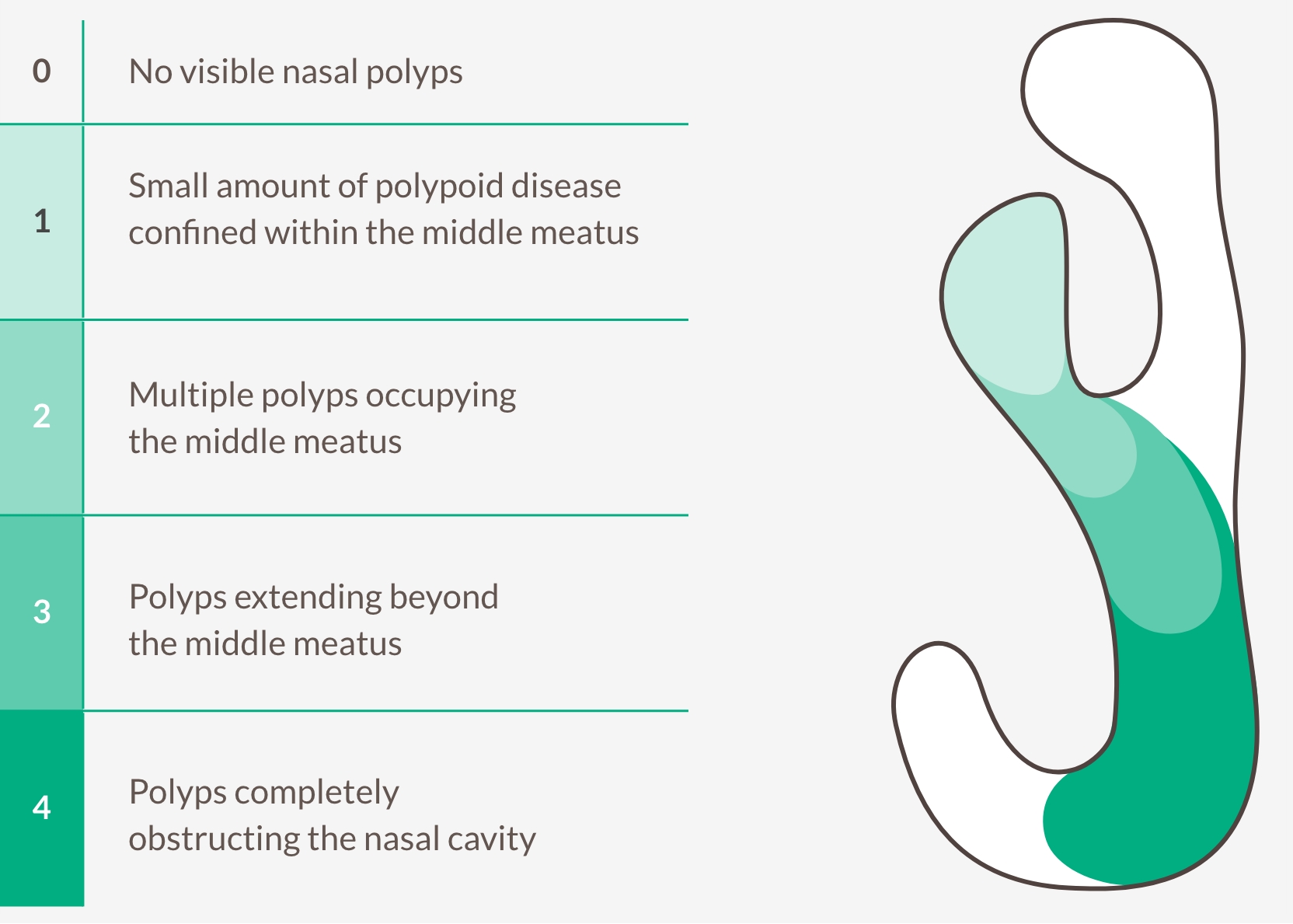

TARGET TYPE 2 INFLAMMATION TO REDUCE NASAL POLYP SIZE AND LIMIT RECURRENCE11,27,28
Bilateral endoscopic nasal polyps score (NPS)
Central-blinded readers grade polyps on the left and right nostril, range 0-4, and assign a score based on the sum of each. The maximum total score is 8. Lower score indicates improvement (smaller polyps).
LMK-CT
TARGET UNDERLYING TYPE 2 INFLAMMATION TO SEE SUSTAINED DECREASES IN SINUS OPACIFICATION1
DUPIXENT significantly decreased LMK-CT scan score vs placebo at Week 24, with greater improvement seen at Week 521
Baseline vs Week 52
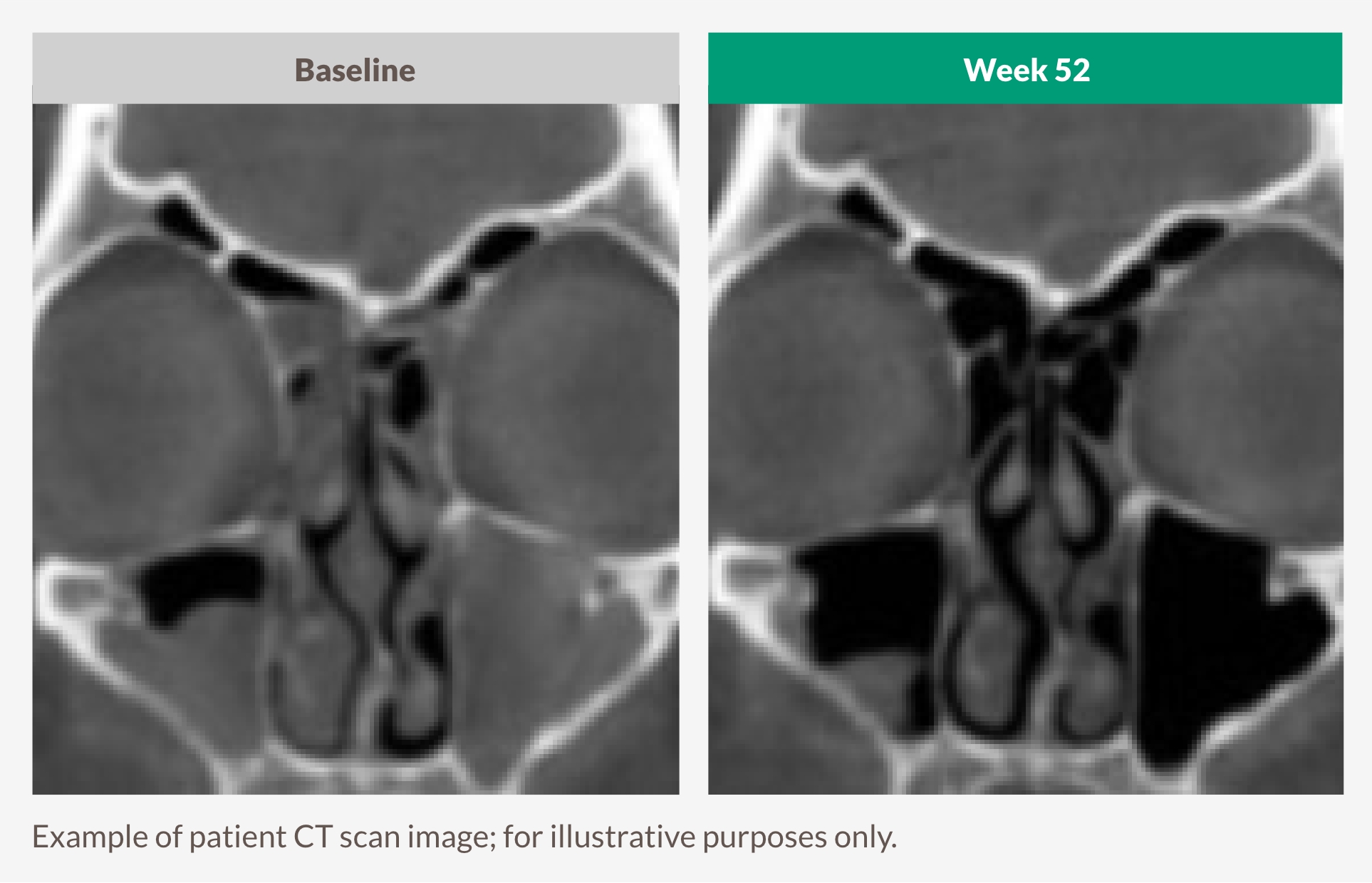

THE ONLY BIOLOGIC TO REPORT LMK-CT RESULTS IN THE APPROVED LABEL1,25,26
5.21 improvement at Week 24 from a baseline score of 18.12 (secondary endpoint) with DUPIXENT 300 mg Q2W + INCS (n=295, pooled DUPIXENT arms) vs 0.09 improvement (from a baseline score of 17.65) with placebo + INCS (n=153) (LSM difference: -5.13 [95% CI: -5.80, -4.46])1
6.83 improvement at Week 52 from a baseline score of 18.42 (secondary endpoint) with DUPIXENT 300 mg Q2W + INCS (n=150) vs 0.11 worsening from a baseline score of 17.65 with placebo + INCS (n=153)(LSM difference: -6.94 [95% CI: -7.87, -6.01])1
Lund-Mackay computed tomography scan (LMK-CT) score
Reader assesses a CT scan of each sinus and assigns a score from 0 (normal) to 2 (complete opacification). For all sinus systems (except the ostiomeatal complex), the total score is the sum from both the right and left sinuses with a maximum of 24. Lower score indicates lower opacification.
Secondary Efficacy
Quality of Life
PATIENTS REPORTED IMPROVED SYMPTOMS AND HEALTH-RELATED QUALITY OF LIFE1,13
DUPIXENT significantly decreased SNOT-22 score vs placebo as early as Week 4, sustained through Weeks 24 and 521,13
Change in SNOT-22 score through Week 52 in SINUS-5213
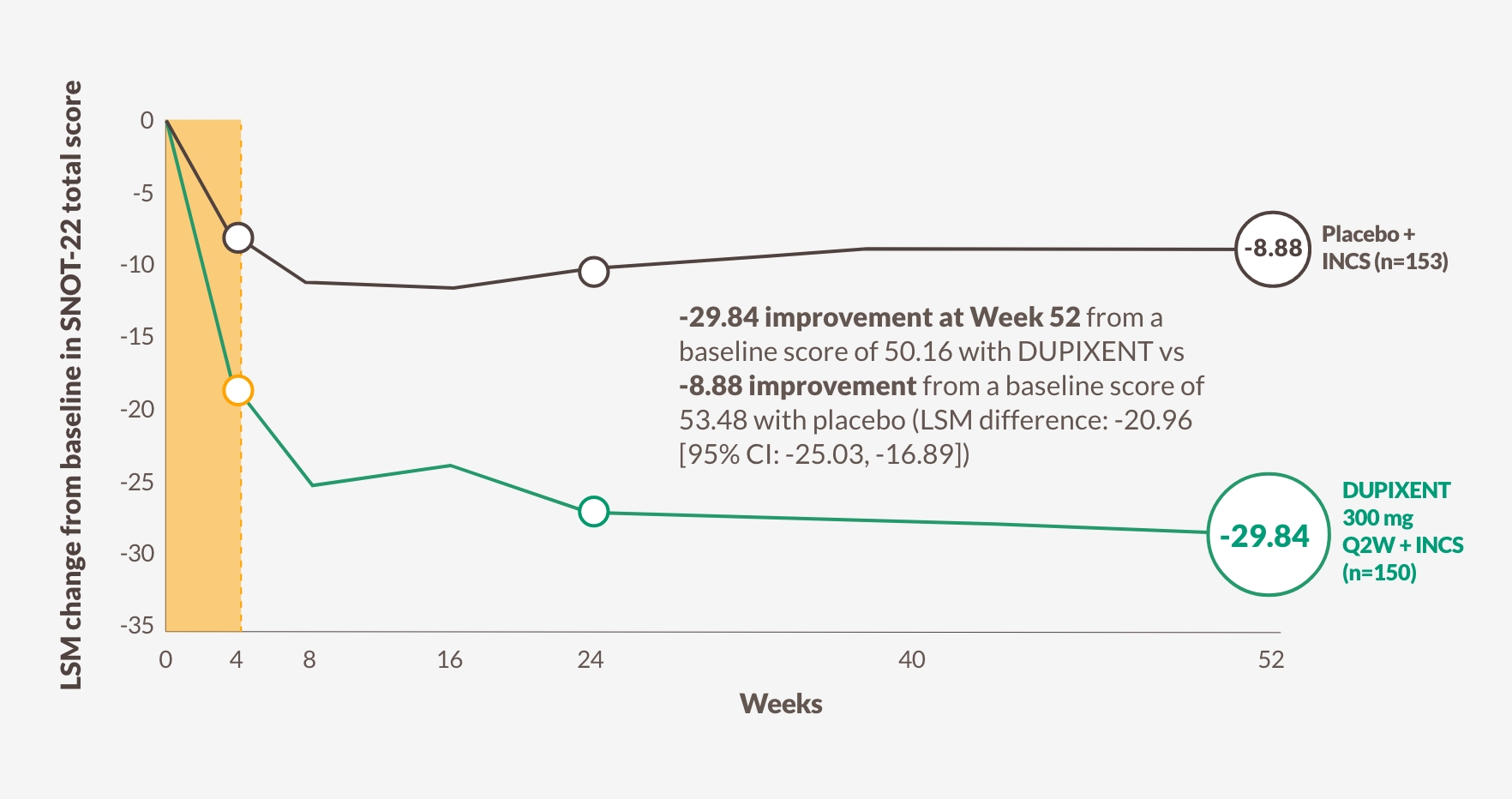
The figure is reproduced by Sanofi based on table S913

DUPIXENT IMPROVED A COMPOSITE SCORE, INCLUDING PHYSICAL SYMPTOMS, SLEEP AND FATIGUE ISSUES, AND PSYCHOSOCIAL LIMITATIONS1,13
The SNOT-22 questionnaire is a patient-reported assessment of 22 symptoms and consequences of CRS with nasal polyps, including29: |
||
|
22-item Sino-Nasal Outcome Test (SNOT-22)
Patients assess 22 symptoms and consequences of CRS with nasal polyps. The maximum score is 110.
The meaningful clinically important difference is 8.9. Lower score indicates better health-related quality of life.
Coexisting Asthma
In patients who have CRS with nasal polyps and coexisting asthma,
DUPIXENT IMPROVED BREATHING AND QUALITY OF LIFE13
DUPIXENT significantly improved lung function and asthma control (ACQ-6) while simultaneously improving CRS with nasal polyps outcomes13
Change in lung function through Week 24 in SINUS-5213
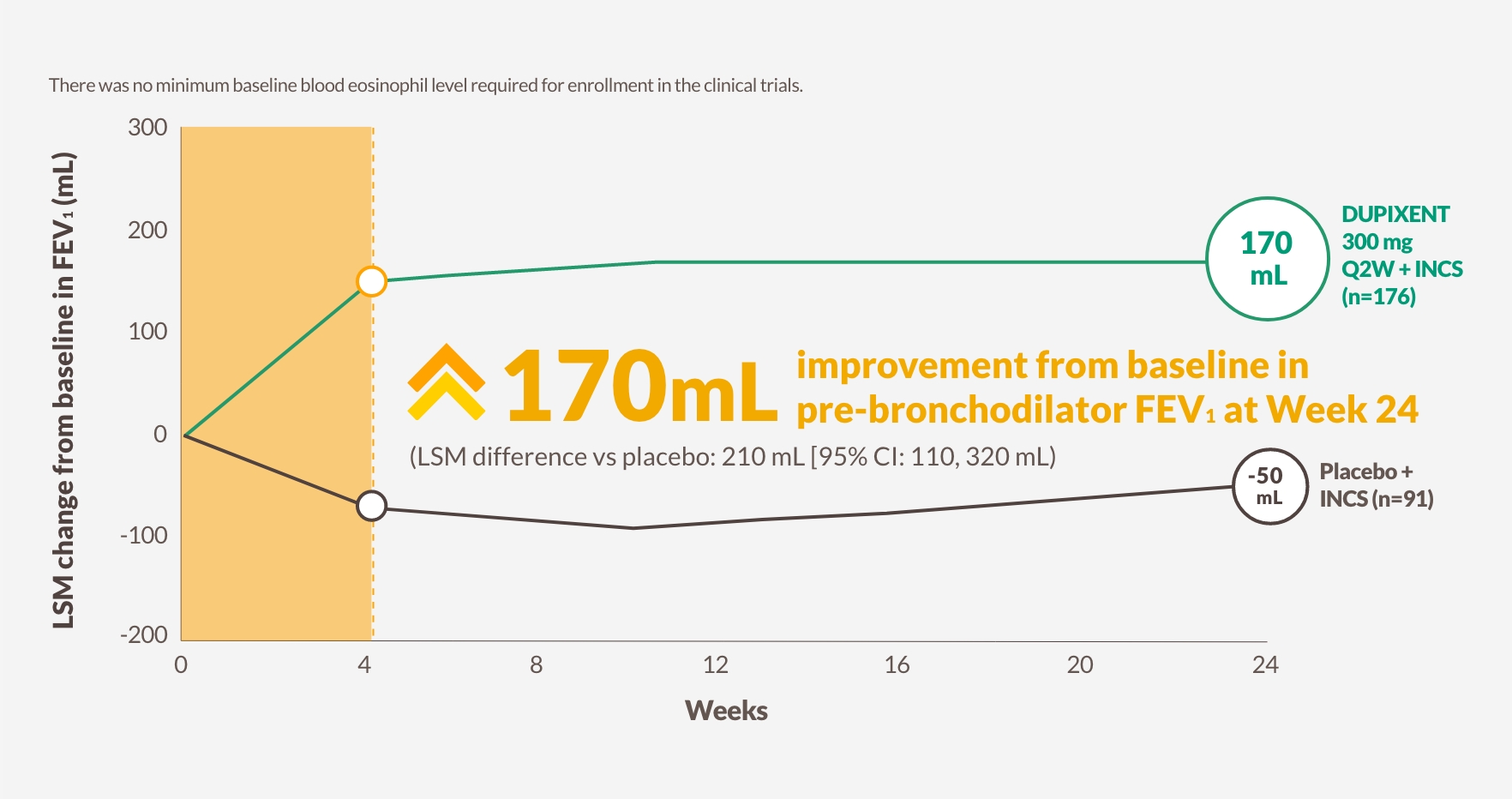
The figure is reproduced by Sanofi based on table S913
Pre-bronchodilator FEV1
170 mL improvement at Week 24 from baseline in pre-bronchodilator FEV1 with DUPIXENT 300 mg Q2W + INCS (n=176, pooled DUPIXENT arms) vs -50 mL worsening with placebo (LSM difference vs placebo: 210 mL [95% CI: 110, 320 mL])13
ACQ-6
-0.78 improvement at Week 24 from a baseline ACQ-6 score of 1.55 with DUPIXENT 300 mg Q2W + INCS (n=176, pooled DUPIXENT arms) vs 0.08 worsening from a baseline score of 1.63 with placebo (LSM difference -0.87 [95% CI: -1.07, -0.66])13
ACQ-6, Asthma Control Questionnaire, 6-item version; FEV1, forced expiratory volume in 1 second.
A patient-reported measure of asthma control (5 questions about symptoms, and 1 question about reliever medication use). The maximum score is 6. The meaningful clinically important difference is 0.5. Lower score indicates better asthma control.
DUPIXENT WAS STUDIED IN THE LARGEST CLINICAL TRIAL PROGRAM IN CRS WITH NASAL POLYPS1,a
Patients were enrolled regardless of prior surgery or systemic steroid use1
|
|
SINUS-24 (N=276) 24 Weeks |
SINUS-52 (N=448) 52 Weeks |
|
Randomized, phase 3 trial |
DUPIXENT + INCS 300 mg Q2W for 24 weeks (n=143) Placebo + INCS for 24 weeks (n=133) |
DUPIXENT + INCS 300 mg Q2W for 52 weeks (n=150)b DUPIXENT + INCS 300 mg Q2W for 24 weeks, followed by Q4W through Week 52 (n=145)c Placebo + INCS for 52 weeks (n=153) |
|
Study population |
>Adults (≥18 years) on background INCS with CRSwNP despite prior sino-nasal surgery or prior treatment with, or who were ineligible to receive or were intolerant to, systemic steroids in the past 2 yearsd >Patients with chronic rhinosinusitis without nasal polyps were not included in these trials >Rescue with systemic steroids or surgery was allowed at investigators’ discretion >The total population of patients in SINUS-24 and SINUS-52 was unrestricted by minimum baseline blood eosinophil count |
|
SINUS-24 Selected Baseline Demographics: Male: 57%; mean CRSwNP duration (SD): 11 (9) years; patients with >1 prior surgery: 72%; patients with systemic steroid use in previous 2 years: 65%; mean bilateral endoscopic NPS (SD), range 0-8: 5.8 (1.3); mean LMK sinus CT total score (SD), range 0-24: 19.0 (4.4); mean UPSIT score (SD), range 0-40: 14.6 (8.5); mean loss of smell score (AM) (SD), range 0-3: 2.7 (0.5); mean SNOT-22 total score (SD), range 0-110: 49.4 (20.2).1
SINUS-52 Selected Baseline Demographics: Male: 62%; mean CRSwNP duration (SD): 11 (10) years; patients with >1 prior surgery: 58%; patients with systemic steroid use in previous 2 years: 80%; mean bilateral endoscopic NPS (SD), range 0-8: 6.1 (1.2); mean LMK sinus CT total score (SD), range 0-24: 18.0 (3.8); mean UPSIT score (SD), range 0-40: 13.6 (8.0); mean loss of smell score (AM) (SD), range 0-3: 2.8 (0.5); mean SNOT-22 total score (SD), range 0-110: 51.9 (20.9).1
a Valid as of August 2023.
b In SINUS-52, data from baseline to Week 24 are pooled DUPIXENT Q2W treatment arms (n=295).
c The recommended dose of DUPIXENT for adult patients with CRSwNP is 300 mg given subcutaneously every other week.
d All patients in the placebo and DUPIXENT arms were on a background therapy of INCS, mometasone furoate nasal spray.
AM, morning; Q4W, once every 4 weeks; SD, standard deviation.
Patient Profiles
IDENTIFY TYPE 2 INFLAMMATION IN PATIENT TYPES LIMITED BY CRS WITH NASAL POLYPS SYMPTOMS2,9
Look for signs of type 2 inflammation and increased disease severity to break the cycle with biologic therapy2,9,30
The different patient types are representative and are not actual DUPIXENT patients.
Signs of type 2 inflammation include:2,9 |
||
|
AERD, Aspirin-Exacerbated Respiratory Disease; NSAID, nonsteroidal antiinflammatory disease.
Safety
ESTABLISHED SAFETY PROFILE WAS CONSISTENT THROUGH WEEK 521
DUPIXENT is a fully human monoclonal antibody with a safety profile comparable to placebo1,a
|
Adverse events in CRSwNP, asthma, and atopic dermatitis clinical trials1,a |
||
|
MedDRA System Organ Class |
Frequencyb |
Adverse reaction |
|
Blood and lymphatic system disorder |
Common |
Eosinophilia |
|
General disorders and administration site conditions |
Common |
Injection site reactions (includes erythema, edema, pruritus, pain, swelling, and bruising)d |
|
Musculoskeletal and connective tissue disorders |
Common |
Arthralgiac |
|
Immune system disorders |
Uncommon Rare |
Angioedemac Anaphylactic reaction Serum sickness reaction Serum sickness-like reaction |
|
Skin and subcutaneous tissue disorders |
Uncommon |
Facial rashc |
|
Adverse events occurring predominantly in atopic dermatitis clinical trials |
||
|
Eye disorders |
Common |
Conjunctivitis allergic |
|
Eye disorders |
Uncommon |
Blepharitisd |
|
Eye disorders |
Rare |
Ulcerative keratitisc,d |
|
Infections and infestations |
Common |
Conjunctivitis |
In the safety pool, the proportion of patients who discontinued treatment due to adverse events:1
2.0% of the DUPIXENT group
4.6% of the placebo group

DUPIXENT IS NOT AN IMMUNOSUPPRESSANT1
a DUPIXENT was studied in 12 randomized, placebo-controlled trials, including atopic dermatitis, asthma, and CRSwNP patients. The pivotal controlled studies involved 4,206 patients receiving dupilumab and 2,326 patients receiving placebo during the controlled period.
b Common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000).
c From postmarketing reporting.
d The frequencies for eye pruritus, blepharitis, and dry eye were common and ulcerative keratitis was uncommon in atopic dermatitis studies.
MedDRA, Medical Dictionary for Regulatory Activities.
Legacy
7-YEAR OF LEGACY ACROSS INDICATIONS DRIVEN BY TYPE 2 INFLAMMATION1,a
Approved across 6 indications driven by type 2 inflammation1

Atopic dermatitis
uncontrolled severeb

Asthma
severe type 2 asthma or OCS-dependentc

CRS with nasal polyps
severe inadequately controlled

Prurigo nodularis
uncontrolled moderate to severe

Eosinophilic esophagitis
inadequately controlled

COPD
uncontrolled while on optimized inhaled therapyd
ATOPIC DERMATITIS
DUPIXENT is indicated for the treatment of moderate-to-severe atopic dermatitis in adults and adolescents 12 years and older who are candidates for systemic therapy.
DUPIXENT is indicated for the treatment of severe atopic dermatitis in children 6 months to 11 years old who are candidates for systemic therapy.
ASTHMA
DUPIXENT is indicated in adults and adolescents 12 years and older as add-on maintenance treatment for severe asthma with type 2 inflammation characterised by raised blood eosinophils and/or raised fraction of exhaled nitric oxide (FeNO), who are inadequately controlled with high dose inhaled corticosteroids (ICS) plus another medicinal product for maintenance treatment.
DUPIXENT is indicated in children 6 to 11 years old as add-on maintenance treatment for severe asthma with type 2 inflammation characterised by raised blood eosinophils and/or raised fraction of exhaled nitric oxide (FeNO), who are inadequately controlled with medium to high dose inhaled corticosteroids (ICS) plus another medicinal product for maintenance treatment.
CRS WITH NASAL POLYPS (CRSwNP)
DUPIXENT is indicated as an add-on therapy with intranasal corticosteroids for the treatment of adults with severe CRSwNP for whom therapy with systemic corticosteroids and/or surgery do not provide adequate disease control.
PRURIGO NODULARIS (PN)
DUPIXENT is indicated for the treatment of adults with moderate-to-severe PN who are candidates for systemic therapy.
EOSINOPHILIC ESOPHAGITIS (EoE)
DUPIXENT is indicated for the treatment of eosinophilic esophagitis in adults and children 1 year or older, weighing at least 15 kg, who are inadequately controlled by, are intolerant to, or who are not candidates for conventional medicinal therapy.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD)
Dupixent is indicated in adults as add-on maintenance treatment for uncontrolled chronic obstructive pulmonary disease (COPD) characterised by raised blood eosinophils on a combination of an inhaled corticosteroid (ICS), a long-acting beta2- agonist (LABA), and a long-acting muscarinic antagonist (LAMA), or on a combination of a LABA and a LAMA if ICS is not appropriate.
For additional information, click here for the SmPC.
a Worldwide across 6 indications.
b In patients aged 6 months to 11 years, DUPIXENT is indicated for severe atopic dermatitis, and DUPIXENT is indicated for moderate-to-severe atopic dermatitis in patients 12+ years.1
c Type 2 asthma patients identified by elevated levels of type 2 biomarkers and OCSdependent patients as specified in GINA guidelines.
d Triple inhaled therapy, or double inhaled therapy if ICS is not appropriate1
GINA, Global Initiative for Asthma; OCS, oral corticosteroid.
Dosing
DUPIXENT OFFERS THE CHOICE OF AT-HOME OR IN-OFFICE ADMINISTRATION1
Recommended dosing for patients with uncontrolled CRS with nasal polyps1
|
18+ YEARS |
300 mga |
every-other-week |
NO LOADING DOSE |
Designed with patients in mind. DUPIXENT has 2 self-administration options1 |
|
Pre-filled Syringe |
Pre-filled Pen |
|
|
|
|
|
DUPIXENT is intended for use under the guidance of a healthcare provider. A patient may self-inject DUPIXENT after training in subcutaneous injection technique using the pre-filled syringe or the pre-filled pen. Provide proper training to patients and/or caregivers on the preparation and administration of DUPIXENT prior to use according to the Instructions for Use.1
DUPIXENT can be administered in the office under the guidance of a healthcare provider if the patient is not an appropriate candidate for self-administration.1
a 300 mg/2 mL solution.
References
1. DUPIXENT Summary of Product Characteristics, 2025.
2. Gandhi NA, Bennet BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35-50. doi:10.1038/nrd4624
3. Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol. 2017;12:331-357. doi:10.1146/annurev-pathol- 052016-100401
4. Stevens WW, Peters AT, Tan BK, et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2019;7(8):2812-2820.e3. doi:10.1016/j.jaip.2019.05.009
5. Maspero J, Adir Y, Al-Ahmad M, et al. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res. 2022;8(3):00576-2021. doi:10.1183/23120541.00576-2021
6. Dietz de Loos DA, Hopkins C, Fokkens WJ. Symptoms in chronic rhinosinusitis with and without nasal polyps. Laryngoscope. 2013;123(1):57-63. doi:10.1002/lary.23671
7. Tsunemi Y, Nakayama T, Kashiwagi T, Akutsu M, Saito S, Haruna S. Long-term efficacy of dupilumab for eosinophilic chronic rhinosinusitis. Am J Rhinol Allergy. 2024;38(1):14-22. doi:10.1177/19458924231204128
8. Han JK, Bosso JV, Cho SH, et al. Multidisciplinary consensus on a stepwise treatment algorithm for management of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2021;11(10):1407-1416. doi:10.1002/alr.22851
9. De Corso E, Baroni S, Settimi S, et al. Sinonasal biomarkers defining type 2-high and type 2-low inflammation in chronic rhinosinusitis with nasal polyps. J Pers Med. 2022;12(8):1251. doi:10.3390/jpm12081251
10. Amirapu S, Biswas K, Radcliff FJ, Wagner Mackenzie B, Ball S, Douglas RG. Sinonasal tissue remodelling during chronic rhinosinusitis. Int J Otolaryngol. 2021;2021:7428955. doi:10.1155/2021/7428955
11. Ferguson BJ, Rizk H, Ramakrishnan J, Pant H. Categorization of nasal polyps. In: Önerci TM, Ferguson BJ, eds. Nasal Polyposis. Springer-Verlag; 2010:103-110.
12. Hellings PW, Peters AT, Chaker AM, et al. Rapid and sustained effects of dupilumab in severe chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2022;12(7):958-962. doi:10.1002/alr.22944
13. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallelgroup phase 3 trials. Lancet. 2019;394(10209):1638-1650. doi:10.1016/S0140- 6736(19)31881-1
14. Canonica GW, Bourdin A, Peters AT, et al. Dupilumab demonstrates rapid onset of response across three type 2 inflammatory diseases. J Allergy Clin Immunol Pract. 2022;10(6):1515-1526. doi:10.1016/j.jaip.2022.02.026
15. Yan X, Whitcroft LK, Hummel T. Olfaction: sensitive indicator of inflammatory burden in chronic rhinosinusitis. Laryngoscope Investig Otolaryngol. 2020;5(6):992-1002. doi:10.1002/lio2.485
16. Chen M, Reed RR, Lane AP. Chronic inflammation directs an olfactory stem cell functional switch from neuroregeneration to immune defense. Cell Stem Cell. 2019;25(4):501-513.e5. doi:10.1016/j.stem.2019.08.011
17. Rouyar A, Classe M, Gorski R, et al. Type 2/Th2-driven inflammation impairs olfactory sensory neurogenesis in mouse chronic rhinosinusitis model. Allergy. 2019;74(3):549-559. doi:10.1111/all.13559
18. Mullol J, Mariño-Sánchez F, Valls M, Alobid I, Marin C. The sense of smell in chronic rhinosinusitis. J Allergy Clin Immunol. 2020;145(3):773-776. doi:10.1016/j.jaci.2020.01.024
19. Bachert C, Bahattacharyya N, Desrosiers M, Khan AH. Burden of disease in chronic rhinosinusitis with nasal polyps. J Asthma Allergy. 2021;14:127-134. doi:10.2147/JAA.S290424
20. DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017;127(3):550-555. doi:10.1002/lary.26391
21. De Corso E, Settimi S, Montuori C, et al. How to manage recurrences after surgery in CRSwNP patients in the biologic era: a narrative review. Acta Otorhinolaryngol Ital. 2023;43(2)(suppl 1):S3-S13. doi:10.14639/0392-100X-suppl.1-43-2023-01
22. Fokkens WJ, Viskens AS, Backer V, et al. EPOS/EUFOREA update on indication and evaluation of biologics in chronic rhinosinusitis with nasal polyps 2023. Rhinology. 2023;61(3):194-202. doi:10.4193/Rhin22.489
23. Hellings PW, Alobid I, Anselmo-Lima WT, Bernal- Sprekelsen M, Bjermer L, Caulley L, Chaker A, Constantinidis J, Conti DM, De Corso E, Desrosiers M. EUFOREA/EPOS2020 statement on the clinical considerations for chronic rhinosinusitis with nasal polyps care. Allergy. 2024 May;79(5):1123-33.
24. Hox V, Lourijsen E, Jordens A, et al. Benefits and harm of systemic steroids for short- and long-term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin Transl Allergy. 2020;10:1. doi:10.1186/s13601-019-0303-6
25. XOLAIR Summary of Product Characteristics, 2020.
26. NUCALA Summary of Product Characteristics, 2021.
27. Han JK, Bachert C, Lee SE, et al. Estimating clinically meaningful change of efficacy outcomes in inadequately controlled chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2022;132(2):265-271. doi:10.1002/lary.29888
28. Gelardi M, Bocciolini C, Notargiacomo M, et al. Chronic rhinosinusitis with nasal polyps: how to identify eligible patients for biologics in clinical practice. Acta Otorhinolaryngol Ital. 2022;42(1):75-81. doi:10.14639/0392-100X-N1699
29. Asiri M, Alokby G. Validation and cross-cultural adaptation of the Sinonasal Outcome Test (SNOT)-22 for the Arabian patient population. Cureus. 2019;11(4):e4447.doi:10.7759/cureus.4447
30. Mullol J, Bachert C, Amin N, et al. Olfactory outcomes with dupilumab in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2022:1086-1095.e5. doi:10.1016/j.jaip.2021.09.037

© 2024 Sanofi and Regeneron Pharmaceuticals. Inc. All Rights Reserved.
MAT-BE-2400891 V1.0 MAY 2025