ISPAD 2023
This year’s edition of the ISPAD conference was a maelstrom of new information, new possibilities, but also, quite naturally, new questions and challenges.
This scientific update will try to summarize some of the most striking sessions of ISPAD 2023 as well as the hopes and puzzlement that they raised.
Watch: Impressions from ISPAD by Lars Krogvold & Riitta Veijola
Impressions from ISPAD
Predictors: Autoimmunity or autoimmunities? And by the way, how do you predict an heterogenous phenomenon?
During two different Sanofi-sponsored symposia, professors Lucienne Chatenoud (Hopital Necker-Enfants Malades, Paris, France), Colin Dayan (Diabetes Research Group, Cardiff University, University Hospital of Wales, Cardiff, UK) and Thomas Danne (Auf der Bult Hospital for Children and Adolescents, Hannover Medical School, Hannover, Germany) reminded us of the etiologies and the natural history of auto-immune type 1 diabetes (aT1D).
Indeed, as illustrated here below, it is widely admitted that environmental events, on a specific genetic background of beta-cell susceptibility or impaired defence mechanisms, will trigger an inappropriate (auto)-immune response against the beta-cells. This T-cell driven immune attack will progressively destroy the beta-cells which will lead to a progressive reduction of insulin secretion inducing dysglycemia (stage 2) and eventually overt symptomatic hyperglycemia (stage 3)1,2.
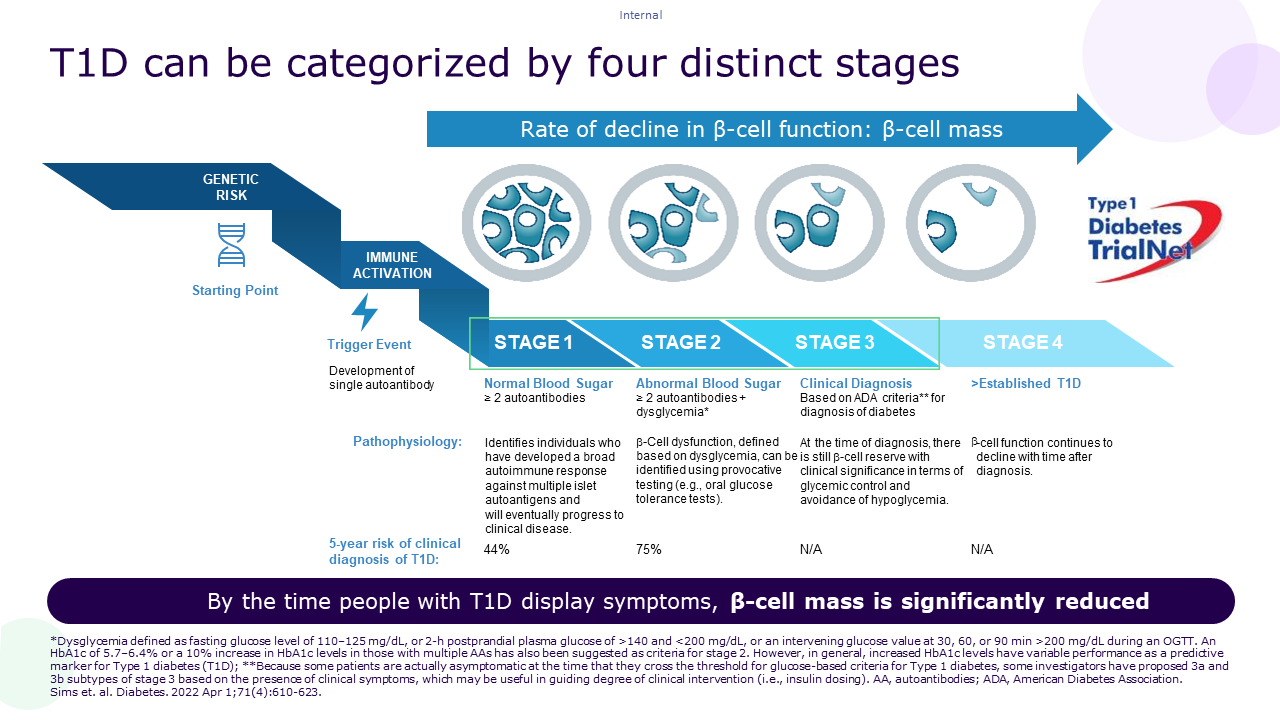
The different stages of aT1D can also be identified by the presence of autoantibodies (Aabs) against beta-cell components (IAA, IA2, GAD65, ZnT8)3.
Understanding the genetic risk factors and the mechanism underlying the induction of the auto-immune reaction as well as identifying predictors and biomarkers of disease stages and progression could allow for the definition of at-risk populations as well as the development of preventive therapies. No wonder thus, that these were predominant themes at ISPAD this year3.
First, in a session dedicated to the prediction of aT1D, prof. Flemming Pociot (Steno Diabetes Center Copenhagen, Denmark) addressed the limitations of Aab testing to predict aT1D progression and called for better markers of the progression of autoimmunity to define responders to clinical trials. Prof. Pociot went on to share data from the Danish National Birth Cohort (538 children) suggesting that hypo-methylation in the promoter region of HLA DRB5 and lipidomics data could predict aT1D at the time of birth (Data available (dnbc.dk)4.
During the same sessions, prof. Max Nieuwdorp (Amsterdam Academic Medical Center, Amsterdam, Netherlands) showed that, even though results on a “microbial signature” of aT1D are conflicting, certain strains were associated with aT1D and reduced Time in Range (TIR)5. He also presented compelling data on the preservation of C-peptide response by Fecal Microbiota Transplantation (FMT) in people with early onset aT1D6,7.
Finally, in a session about prediction and epidemiology of aT1D, Dr Roos Van Rhijn established a profile of Aab negative children with aT1D. This profile concerns 10% of children living with aT1D and is characterized by a later age at onset, a higher prevalence in non-caucasian youth, a lesser association with other auto-immune diseases and family history of aT1D, a lesser incidence of DKA and slower beta-cell destruction 8,9.
This highlights the heterogeneity of the auto-immune phenomenon in aT1D and reflects on the call of prof. Pociot for additional biomarkers as well as the growing interest and need for precision medicine guidelines10.
Watch: The different stages of T1D and why it is relevant with Flemming Pociot & Henk-Jan Aanstoot
Screening: Who is being screened where? A snapshot of screening initiatives
Primary results from both the Diaunion and INNODIA screening programs were shared at ISPAD this year. Prof. Daniel Agardh (Lund University, Lund, Sweden) presented Diaunion, a collaboration between Lund University and the Steno Diabetes Center in Copenhagen for early detection of aT1D, celiac disease and autoimmune thyroid disease. The 3 diseases have indeed been shown to co-occur and one of the aims of the Diaunion Triad study (to be completed in 2025; Screening for type 1 diabetes, celiac disease and thyroiditis in children: TRIAD study — Lund University) is to provide data on the prevalence of celiac disease (CD), auto-immune thyroid disease (AIT) and aT1D in the general population as well as in first degree relatives (FDR) of people with aT1D11-13.
Briefly, between September and December 2021, 10.000 children between 6-8 years and 13-15 years of age were randomly selected and invited to take a capillary blood sample at home. Among the blood samples analyzed, 15.5% were positive for any of the seven autoantibodies tested. Extrapolating the autoantibodies results as a proxy for ongoing disease autoimmunity, 3.5% had aT1D autoimmunity and 0.8% had autoimmunity for two diseases. In the 6-8-year-old age group, autoantibodies for CD were most prevalent while in the 13-15-year-old age group it was AIT autoimmunity. There were more children with a first degree relative with one or more of the triad diseases in the group of children who were positive for at least 1 autoantibody compared to the entire tested group14.
The next day, dr. Loredana Marcovecchio (University of Cambridge, Cambridge, UK), presented data from the INNODIA cohort of first degree relatives of people with type 1 diabetes (For people with an increased Type 1 Diabetes risk - INNODIA). They found that the prevalence of single positive autoantibody increased with age but that the younger people in the cohort positive for autoantibodies presented lower stimulated C-peptide values which dovetails data from the literature15. Interestingly, the INNODIA team collected Continuous Glucose Monitoring (CGM) data as part of the follow up monitoring of positive children to compare the predictive value to that of oral glucose tolerance testing (OGTT), a more invasive although highly sensitive technique16,17.
Watch: Thoughts about screening by Flemming Pociot & Henk-Jan Aanstoot
Treatment: People with stage 1, 2 or 3: what can we propose to them?
During the Sanofi-sponsored symposia, entitled “Exploring advancements in the aT1D treatment landscape: Is there a future for disease-modifying therapies?” and “The Now Known: Disrupting the pathogenesis of T1D”, professors Colin Dayan (Diabetes Research Group, Cardiff University, University Hospital of Wales, Cardiff, UK) and Kimber Simmons (Barbara Davis Center for Diabetes, University of Colorado, Boulder, Colorado, USA) enlightened us about emerging disease-modifying therapies in aT1D.
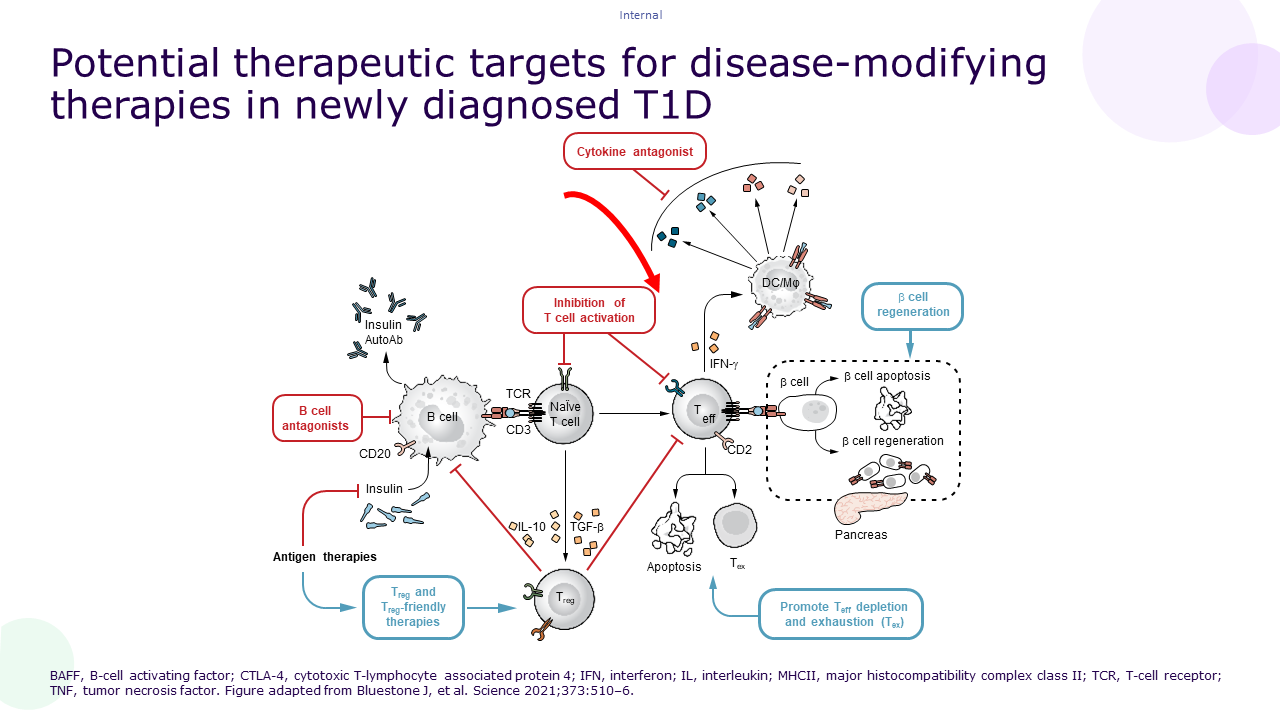
Indeed, slowing or halting the autoimmune process with disease-modifying therapies could allow for beta-cell mass preservation and subsequently delay the progression through the different stages of the disease. Ultimately, this could preserve insulin secretion and delay or reduce the need for insulin therapy18.To quote prof. Chantal Mathieu (Clinical and Experimental Endocrinology, KU Leuven, Leuven, Belgium): “Disease modifying therapy may have the potential to influence the course of aT1D and offer an additional approach to insulin-only management.”
Several disease-modifying therapies are currently under investigation for the treatment of aT1D19.
Why should we do this?: So what? What is the added value of screening, delaying onset and preserving beta-cell function for people?
The therapies discussed in the section "Treatment" could fill the unmet need for the development of therapies that could be used in addition to symptomatic treatments20.
Indeed, as discussed by prof. Mathieu and despite significant advances in diabetes management, the majority (~70%) of people with aT1D do not achieve adequate glycemic control21.
Preserving beta-cell function early after diagnosis of stage 3 could improve glycemic control, decrease hypoglycemia and lower the incidence of retinopathy and nephropathy22,23. Even earlier interventions that delay the onset of stage 3 aT1D may reduce hospital visits, the burden of insulin treatment, the duration of diabetes and the psychological burden of the disease24. Speaking of psychological burden, evidence brought up by Sanjoy Dutta (JDRF, New York, USA) shows that youth with aT1D have a twice higher risk to suffer from psychiatric disorders and thus have a lower quality of life. Furthermore, having a child diagnosed with aT1D profoundly affects parents and siblings with 24% of mothers meeting the diagnoses criteria of Post-traumatic Stress and family members being at high risk of anxiety and depression25,26.
If preserving beta-cell function early after diagnosis and delaying onset of stage 3 diabetes is the (near) future, screening initiatives are ongoing and generating benefits of their own for the people presenting Aabs as well as for their families. Indeed, screening and monitoring have shown to be associated with reduction of parental and child stress at diagnosis, an opportunity for better psychological adjustment to the diagnosis and reductions of Diabetic Keto Acidosis (DKA) at diagnosis of up to 90%.27
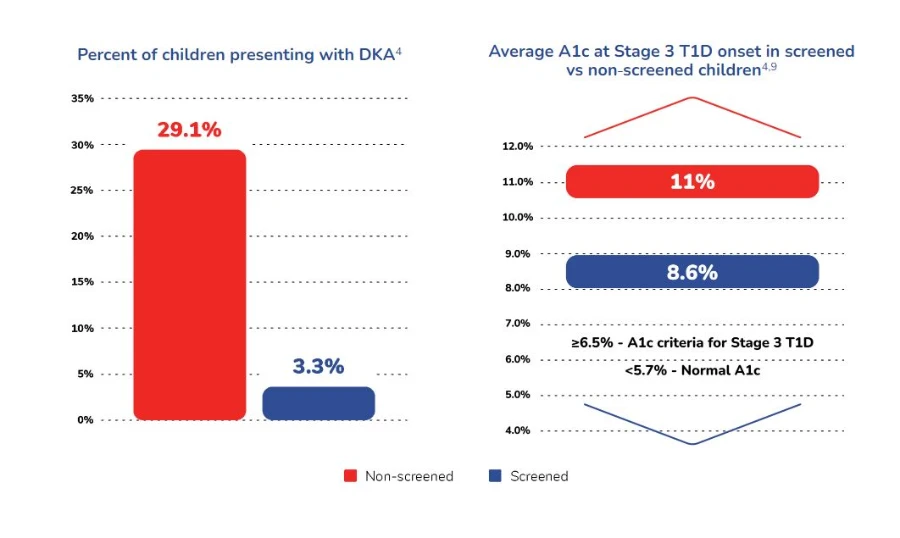
Winkler C, Schober E, Ziegler A-G, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes. 2012;13(4):308-313.
This reduction of DKA incidence is particularly relevant for children’s health as subclinical brain injuries have been observed following DKA cases28. In a nutshell, the positive impact of screening could (and has) been described as allowing for a “soft landing” in stage 3 aT1D for the children and their families.
Finally, and most importantly, as brilliantly highlighted during the People Reported Outcomes (PRO) session by Marissa Town, diabetes nurse and person living with diabetes, there is no one-size-fits-all approach to diabetes care. Therefore, considering the unique needs and preferences of each person living with diabetes and including them in the design of care models and trials to ensure that their perspectives are considered is primordial.
Video: Take home messages from your colleagues
1. Besser REJ, Bell KJ, Couper JJ, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Stages of type 1 diabetes in children and adolescents. Pediatr Diabetes. 2022;23(8):1175-1187. doi:10.1111/pedi.13410
2. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964-1974. doi:10.2337/dc15-1419
3. Sims EK, Besser REJ, Dayan C, et al. Screening for Type 1 Diabetes in the General Population: A Status Report and Perspective. Diabetes. 2022;71(4):610-623. doi:10.2337/dbi20-0054
4. Olsen J, Melbye M, Olsen SF, et al. The Danish National Birth Cohort--its background, structure and aim. Scand J Public Health. 2001;29(4):300-307. doi:10.1177/14034948010290040201.
5. Zhang L, Jonscher KR, Zhang Z, et al. Islet autoantibody seroconversion in type-1 diabetes is associated with metagenome-assembled genomes in infant gut microbiomes. Nat Commun. 2022;13(1):3551. Published 2022 Jun 21. doi:10.1038/s41467-022-31227-1
6. Groen AK, Nieuwdorp M. An evaluation of the therapeutic potential of fecal microbiota transplantation to treat infectious and metabolic diseases. EMBO Mol Med. 2017;9(1):1-3. doi:10.15252/emmm.201607035
7. de Groot P, Nikolic T, Pellegrini S, et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut. 2021;70(1):92-105. doi:10.1136/gutjnl-2020-322630
8. Patel SK, Ma CS, Fourlanos S, Greenfield JR. Autoantibody-Negative Type 1 Diabetes: A Neglected Subtype. Trends Endocrinol Metab. 2021;32(5):295-305. doi:10.1016/j.tem.2021.02.001
9. Kobayashi M, Ohara N, Ikeda Y, et al. Glutamic Acid Decarboxylase Autoantibody-negative Slowly Progressive Type 1 Diabetes Mellitus: A Case Report and Literature Review. Intern Med. 2018;57(24):3581-3587. doi:10.2169/internalmedicine.1008-18
10. Redondo MJ, Morgan NG. Heterogeneity and endotypes in type 1 diabetes mellitus. Nat Rev Endocrinol. 2023;19(9):542-554. doi:10.1038/s41574-023-00853-0
11. Pham-Short A, Donaghue KC, Ambler G, Phelan H, Twigg S, Craig ME. Screening for Celiac Disease in Type 1 Diabetes: A Systematic Review. Pediatrics. 2015;136(1):e170-e176. doi:10.1542/peds.2014-2883
12. Kochummen E, Marwa A, Umpaichitra V, Perez-Colon S, Chin VL. Screening for autoimmune thyroiditis and celiac disease in minority children with type 1 diabetes. J Pediatr Endocrinol Metab. 2018;31(8):879-885. doi:10.1515/jpem-2017-0254
13. Michael Freemark, Lynne L. Levitsky; Screening for Celiac Disease in Children With Type 1 Diabetes : Two views of the controversy. Diabetes Care 1 June 2003; 26 (6): 1932–1939. https://doi.org/10.2337/diacare.26.6.1932
14. Diaunion Status report 15 October 2020 to 31 December 2021: Research – DiaUnion. Last accessed 26/10/2023.
15. Redondo MJ, van Raalte DH. Age Ain't Nothing But a Number . . . or Is It?. Diabetes Care. 2023;46(6):1135-1136. doi:10.2337/dci23-0013
16. Wilson DM, Pietropaolo SL, Acevedo-Calado M, et al. CGM Metrics Identify Dysglycemic States in Participants From the TrialNet Pathway to Prevention Study. Diabetes Care. 2023;46(3):526-534. doi:10.2337/dc22-1297
17. Steck AK, Dong F, Geno Rasmussen C, et al. CGM Metrics Predict Imminent Progression to Type 1 Diabetes: Autoimmunity Screening for Kids (ASK) Study. Diabetes Care. 2022;45(2):365-371. doi:10.2337/dc21-0602
18. Jeffrey A. Bluestone et al. Immunotherapy: Building a bridge to a cure for type 1 diabetes.Science373,510-516(2021).DOI:10.1126/science.abh1654
19. Simmons KM, Sims EK. Screening and prevention of type 1 diabetes: Where are we? [published online ahead of print, 2023 Jun 8]. J Clin Endocrinol Metab. 2023;dgad328. doi:10.1210/clinem/dgad328
19. Coppieters K, von Herrath M. The Development of Immunotherapy Strategies for the Treatment of Type 1 Diabetes. Front Med (Lausanne). 2018;5:283. Published 2018 Oct 9. doi:10.3389/fmed.2018.00283
20. Greenbaum C, VanBuecken D, Lord S. Disease-Modifying Therapies in Type 1 Diabetes: A Look into the Future of Diabetes Practice. Drugs. 2019;79(1):43-61. doi:10.1007/s40265-018-1035-y
21. Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne). 2013;4:37. Published 2013 Mar 27. doi:10.3389/fendo.2013.00037
22. Palmer JP, Fleming GA, Greenbaum CJ, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001 [published correction appears in Diabetes. 2004 Jul;53(7):1934]. Diabetes. 2004;53(1):250-264. doi:10.2337/diabetes.53.1.250
23. Quinn LM, Swaby R, Tatovic D, Narendran P, Besser REJ, Dayan CM. What does the licensing of teplizumab mean for diabetes care?. Diabetes Obes Metab. 2023;25(8):2051-2057. doi:10.1111/dom.15071
24. de Wit M, Gajewska KA, Goethals ER, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Psychological care of children, adolescents and young adults with diabetes. Pediatr Diabetes. 2022;23(8):1373-1389. doi:10.1111/pedi.13428
25. Whittemore R, Jaser S, Chao A, Jang M, Grey M. Psychological experience of parents of children with type 1 diabetes: a systematic mixed-studies review. Diabetes Educ. 2012;38(4):562-579. doi:10.1177/0145721712445216
26. Besser REJ, Ng SM, Gregory JW, Dayan CM, Randell T, Barrett T. General population screening for childhood type 1 diabetes: is it time for a UK strategy?. Arch Dis Child. 2022;107(9):790-795. doi:10.1136/archdischild-2021-321864
27. Glaser N, Fritsch M, Priyambada L, et al. ISPAD clinical practice consensus guidelines 2022: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2022;23(7):835-856. doi:10.1111/pedi.13406








