Role of type 2 Inflammation*
* Type 2 inflammation defined as elevated biomarker in COPD (Blood EOS ≥300 cells/μL or ≥2%, or Sputum EOS ≥ 3%)
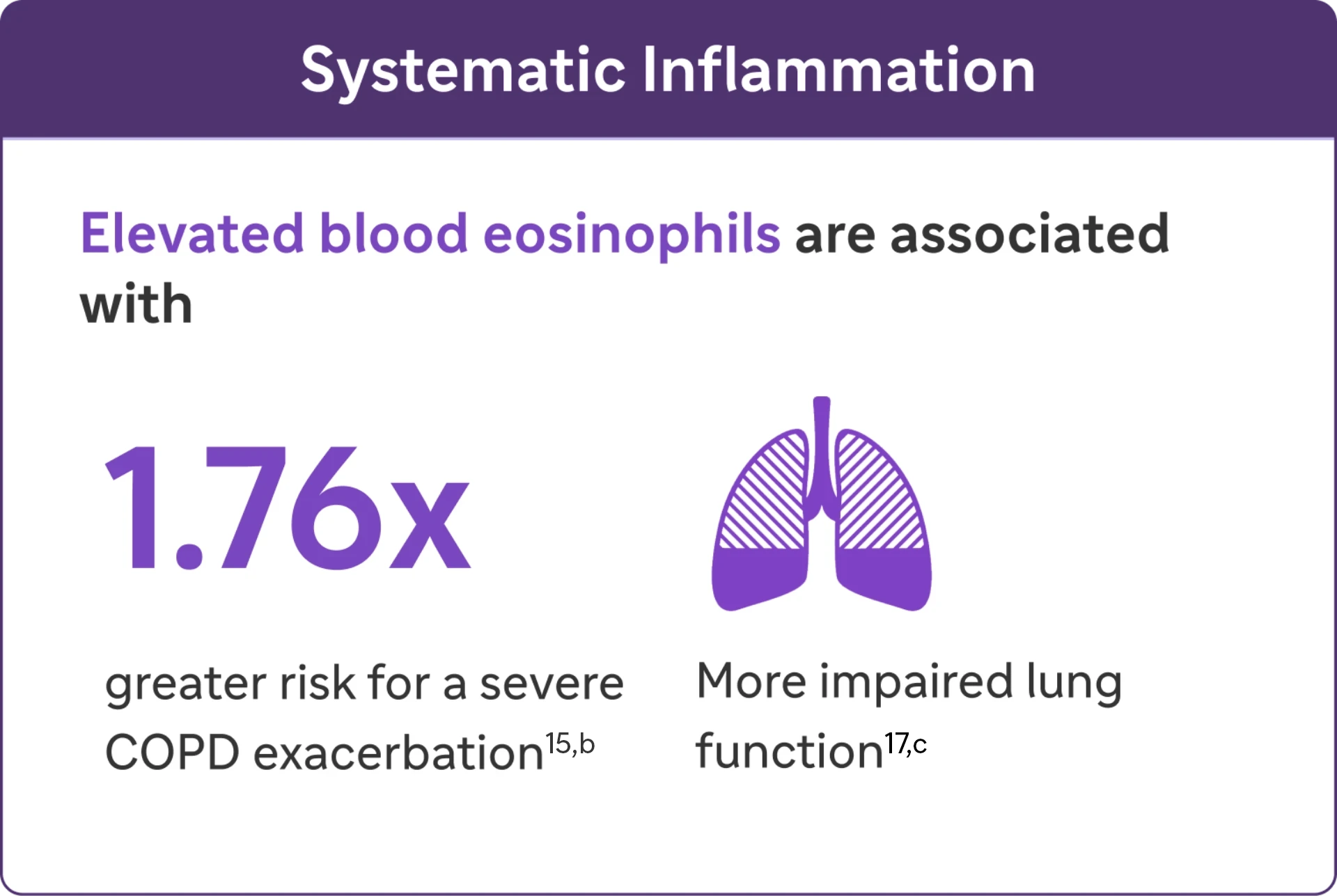
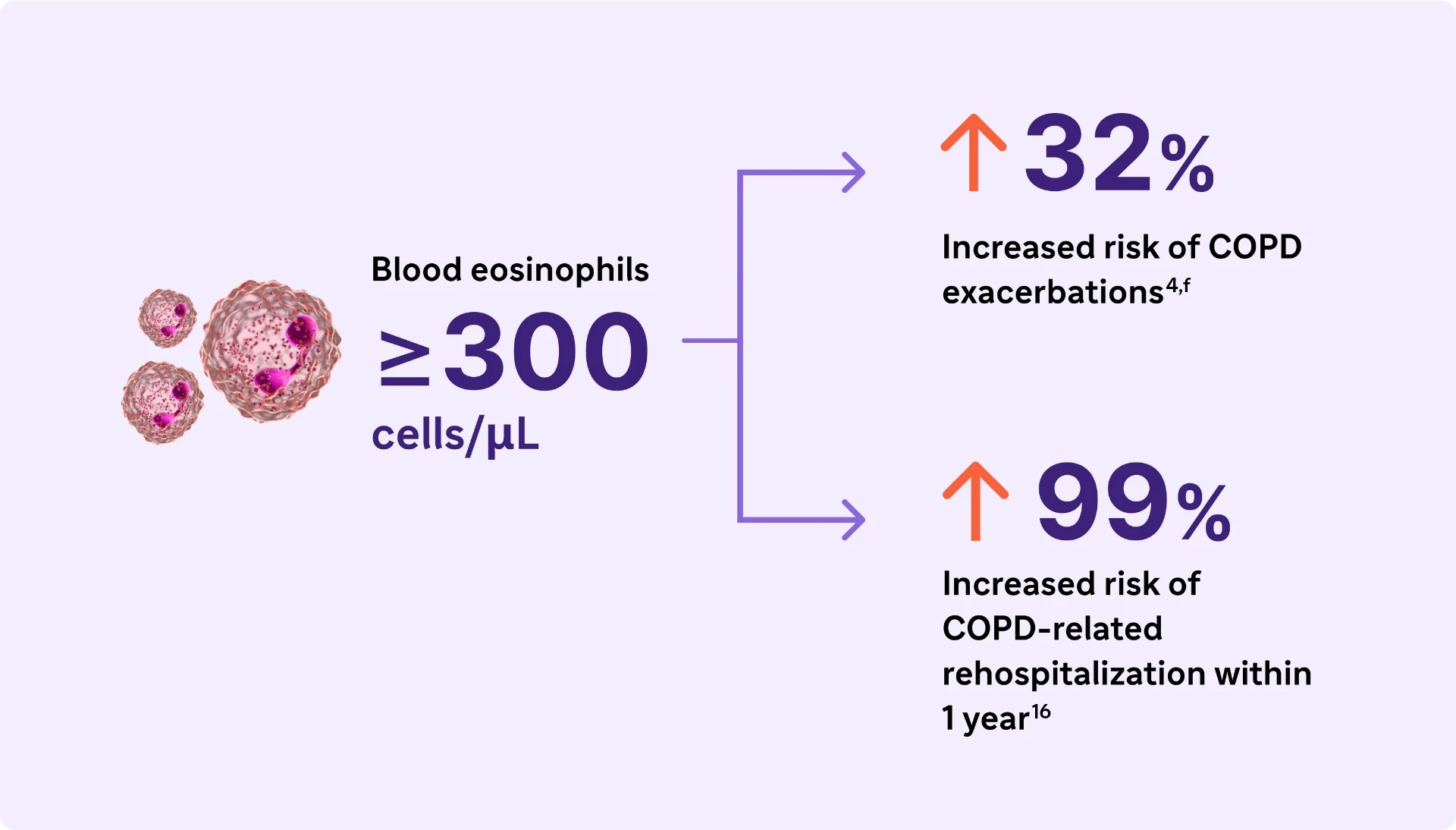
Type 2 Inflammation* may increase the risk of exacerbations and lung function impairment in COPD4,16
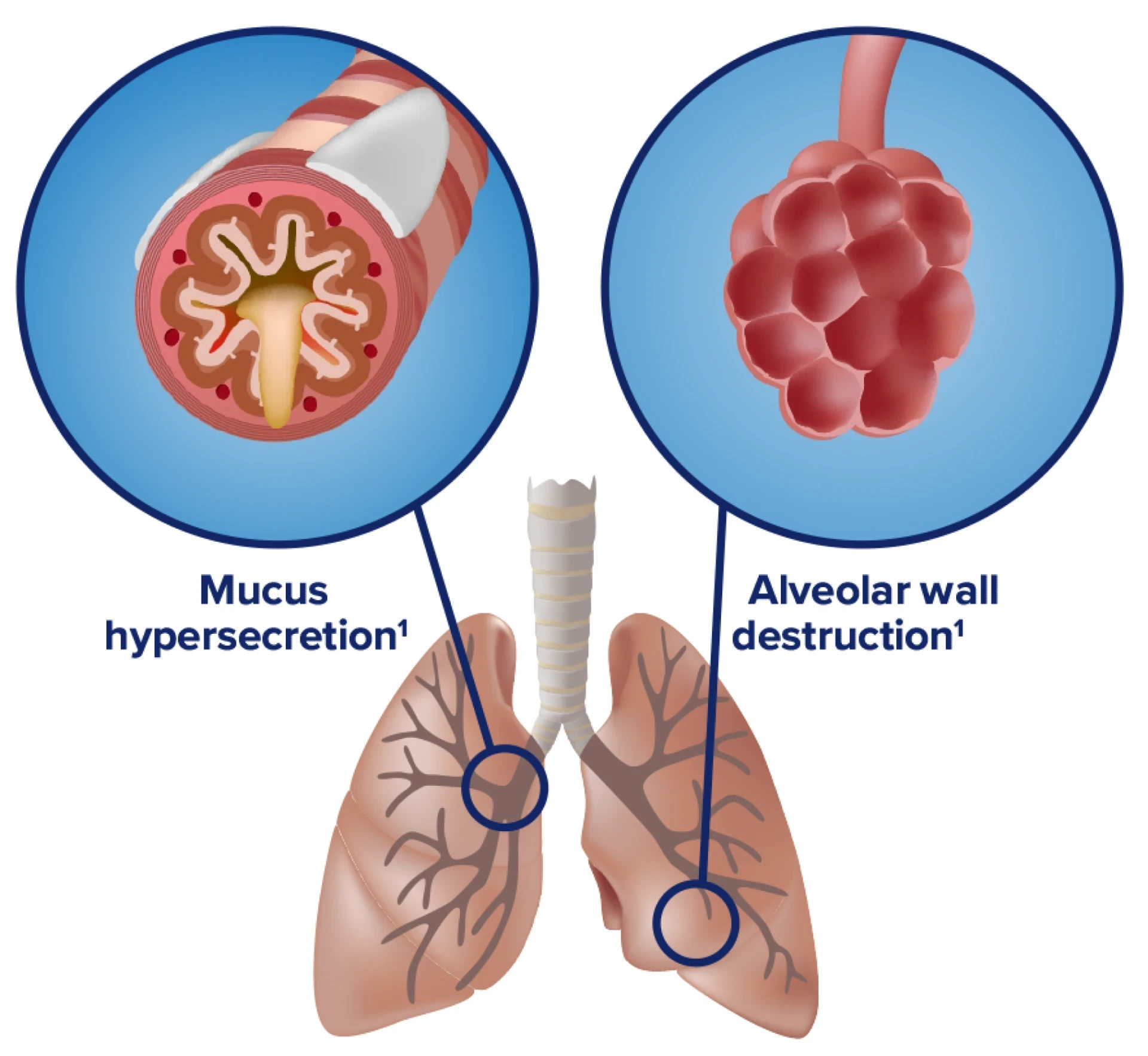
COPD is characterized by mucus production, airway obstruction, and coughing1
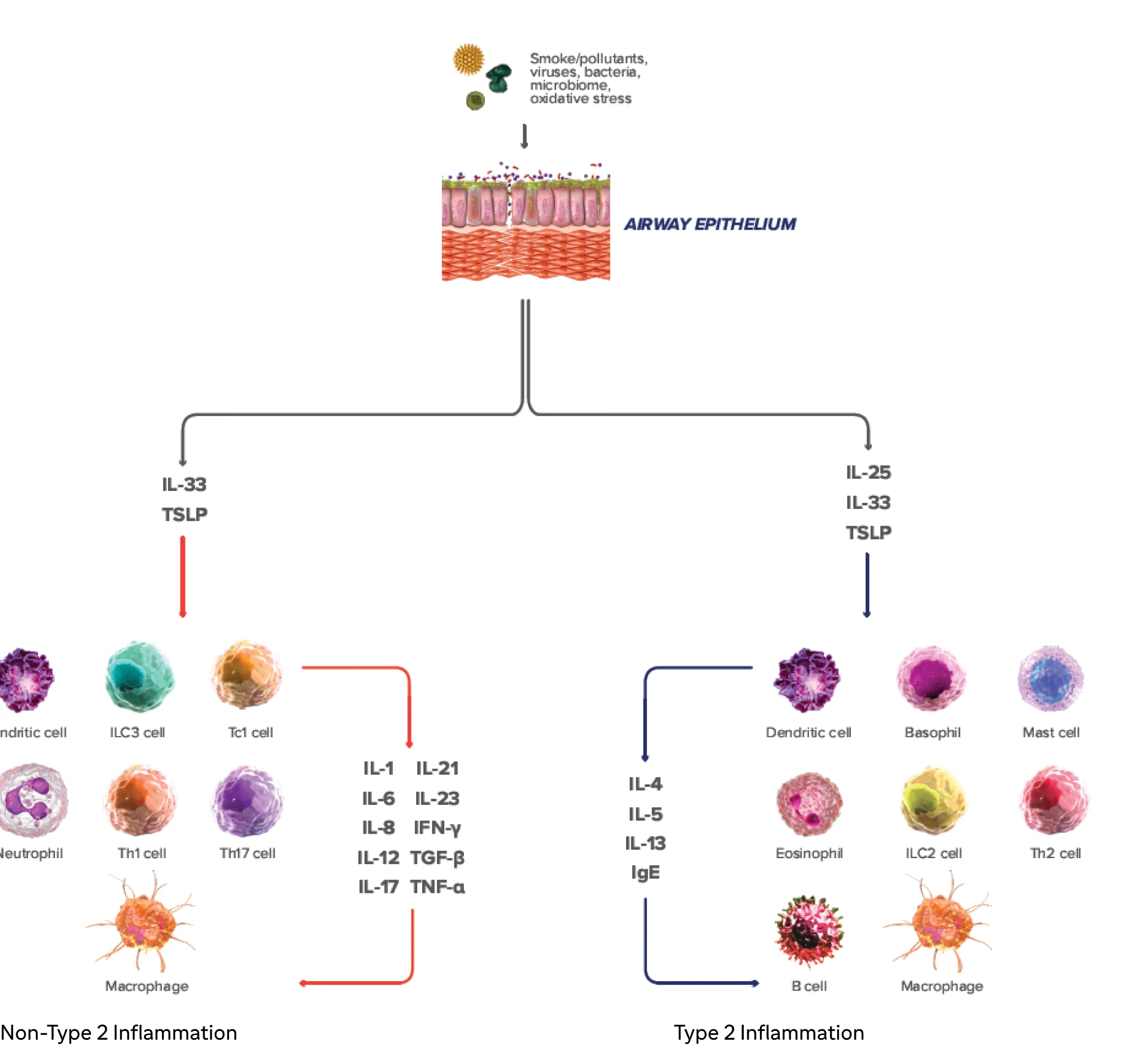
Inflammation manifests systemically and locally
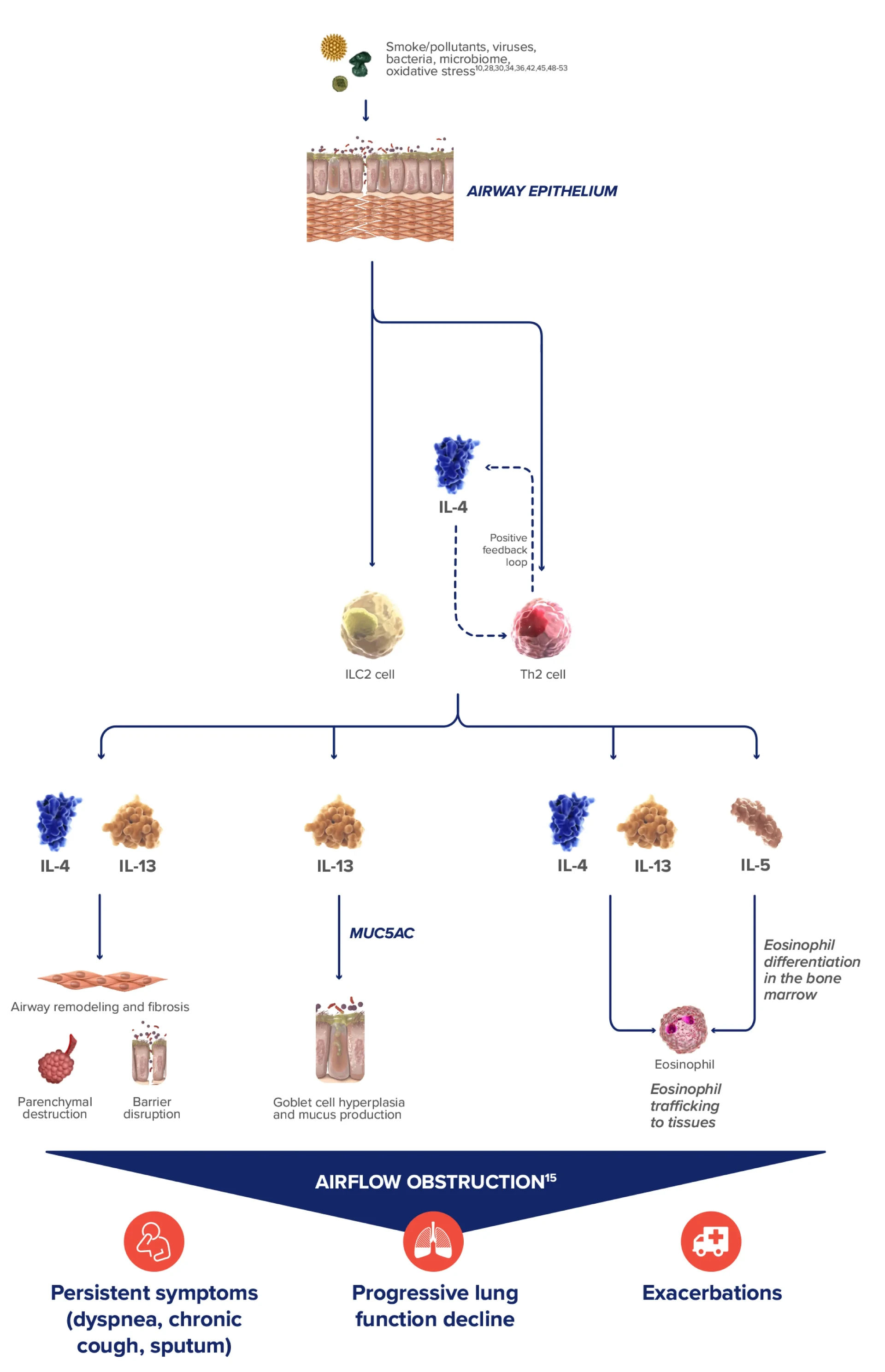
- Chronic inflammation causes structural changes, including narrowing of the airways and decreased lung elasticity1
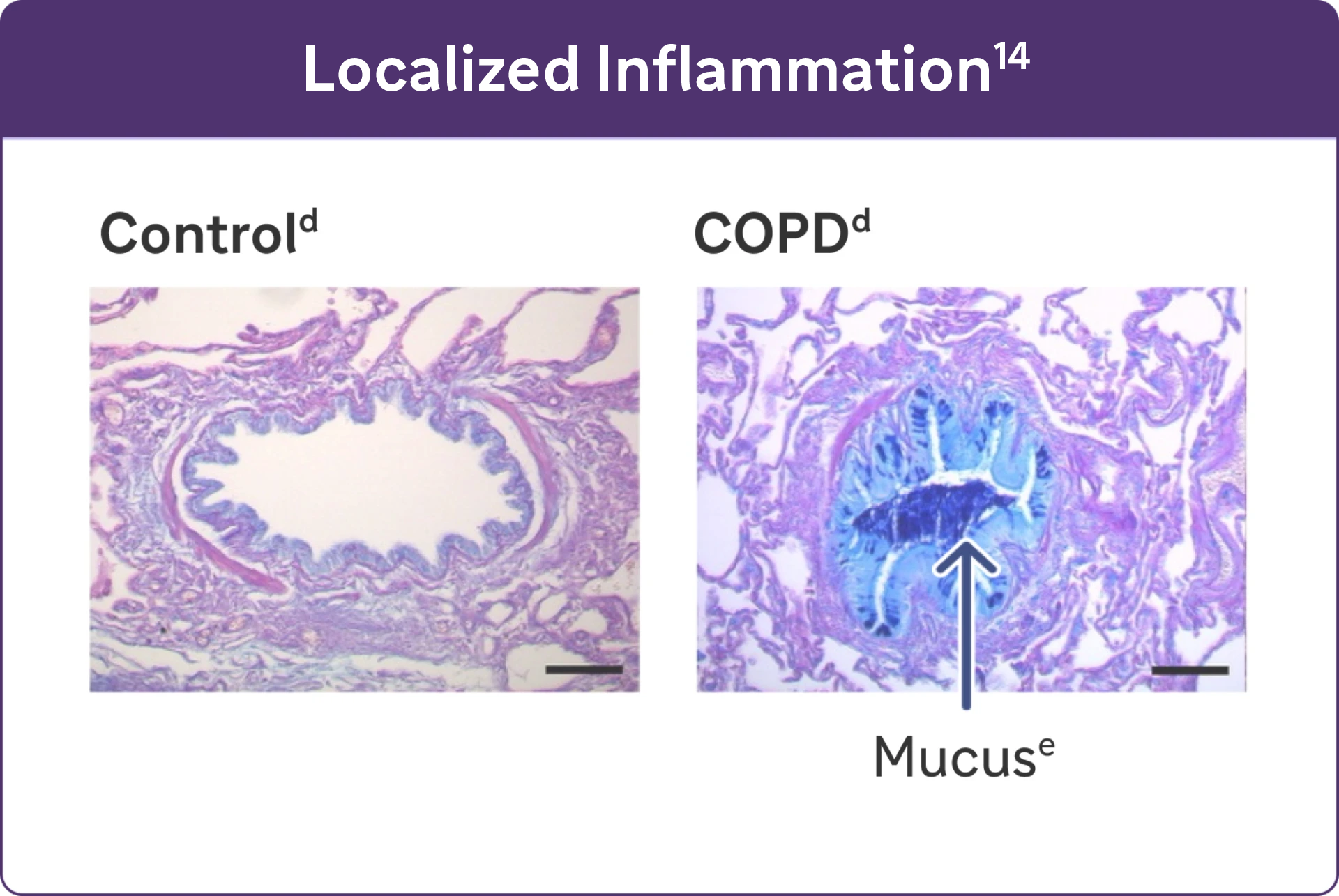
- Studies have identified a relationship between inflammation and mucus hypersecretion in respiratory conditions such as COPD14
* Type 2 inflammation defined as elevated biomarker in COPD (Blood EOS ≥300 cells/μL or ≥2%, or Sputum EOS ≥ 3%)
Look for elevated blood eosinophils (>300 Cells/μL) - A Biomarker of Type 2 Inflammation* in COPD1
Elevated eosinophils in COPD are a treatable trait and marker of type 2 inflammation*1
The 2024 GOLD Report recognizes elevated blood EOS as a clinically used biomarker in identifying COPD with type 2 inflammation*.1
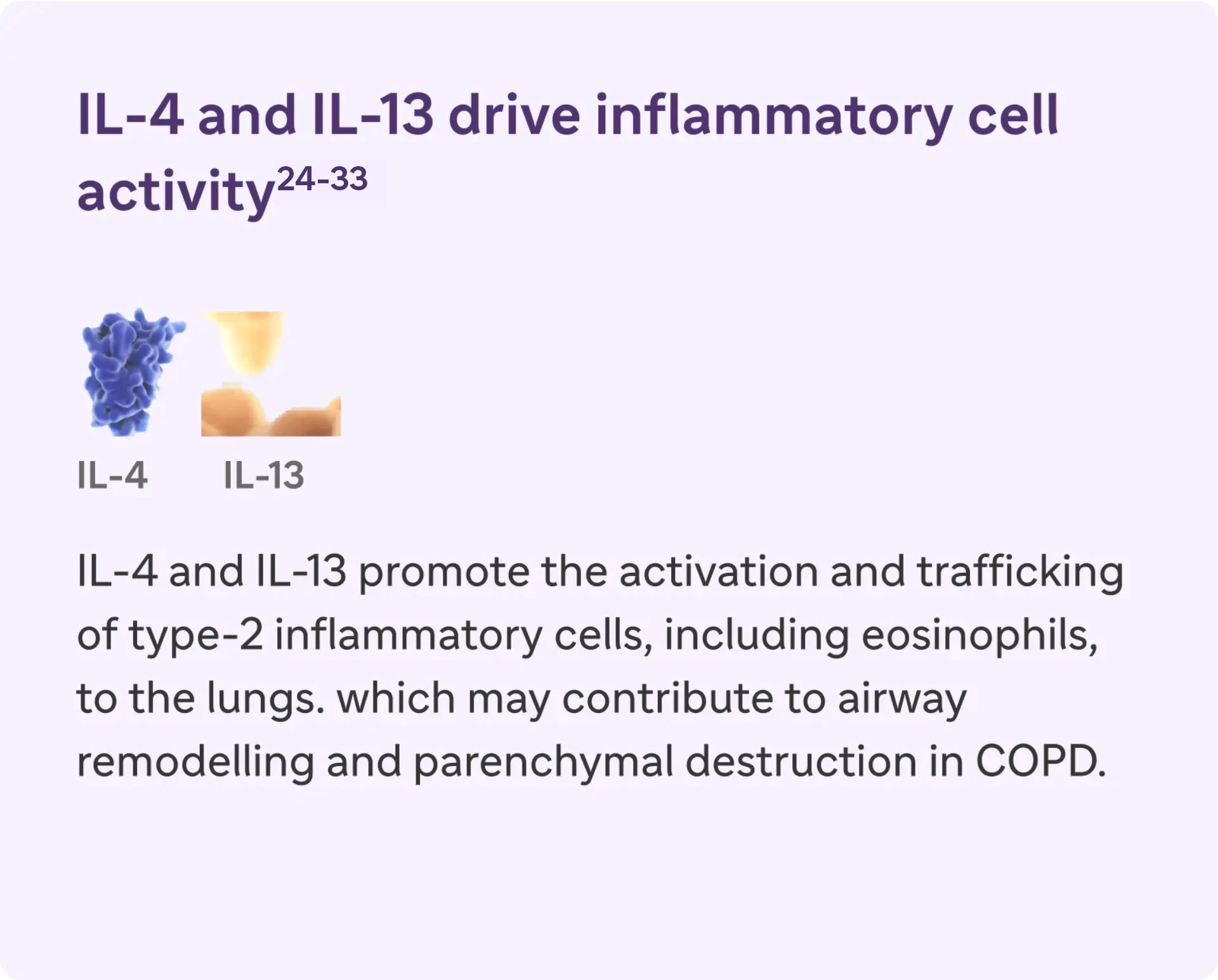
Type 2 inflammation* in COPD can involve multiple pathways, cytokines, and inflammatory cells18-23
IL-4, IL-13, an IL-5 are type 2 cytokines involved in COPD
* Type 2 inflammation defined as elevated biomarker in COPD (Blood EOS ≥300 cells/μL or ≥2%, or Sputum EOS ≥ 3%)
aA severe exacerbation was defined as a hospitalization due to COPD. Exacerbations had to be a minimum of 4 weeks apart to be considered separate exacerbations.27
bIn a cohort of patients with EOS >200 cells/μL.26
cReproduced with permission of the American Thoracic Society. Fritzsching B et al. Am J Respir Crit Care Med. 2015;191(8):902-91325
dAlcian blue PAS staining of mucus in airway epithelial cells.25
eResults from an observational study of 1553 patients with GOLD spirometry grade 2-4 COPD (postbronchodilator FEV1/FVC ratio <0.7, with FEV, >80% predicted)23
fResults from a 1-year observational study of 479 patients with COPD, 173 of whom had blood eosinophil levels ≥200 cells/μL and/or ≥2% of the total white blood cell count.24
COPD, chronic obstructive pulmonary disease; EOS, eosinophils; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IFN-y, interferon-gamma; ILC2, innate lymphoid type-2 cells; MUC5AC, mucin 5AC; PAS, periodic acid-Schiff; TNF-a, tumor necrosis factor alpha; TSLP, thymic stromal lymphopoietin.
-
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2024 report). Accessed [February 9, 2024]. https://goldcopd.org/2024-gold-report-2/
-
Halpin DMG, Dransfield MT, Han MK, et al. The effect of exacerbation history on outcomes in the IMPACT trial. Eur Respir J. 2020;55:1901921. doi:10.1183/13993003.01921-2019
-
Casanova C, Celli BR, de-Torres JP, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J. 2017;50:1701162. doi:10.1183/13993003.01162-2017
-
Yun JH, Lamb A, Chase R, et al; COPDGene and ECLIPSE Investigators. Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141(6):2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
-
Oshagbemi OA, Franssen FME, van Kraaij S, et al. Blood eosinophil counts, withdrawal of inhaled corticosteroids and risk of COPD exacerbations and mortality in the clinical practice research datalink (CPRD). COPD. 2019;16(2):152-159.
-
Oshagbemi OA, Burden AM, Braeken DCW, et al. Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am J Respir Crit Care Med. 2017;195(10):1402-1404.
-
Ajithkumar CS 2018
-
Wu HX, et al. Prevalence and Baseline Clinical Characteristics of Eosinophilic Chronic Obstructive Pulmonary Disease: A Meta-Analysis and Systematic Review. Front Med (Lausanne). 2019; 6:282.
-
Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662-671 (Leigh 2006)
-
Leigh R,et al. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. 2006;27:964–971.
-
Schumann DM, et al. Stability of the Blood Eosinophilic Phenotype in Stable and Exacerbated COPD. Chest. 2019; 156:456–465.
-
Jamieson DB, et al. Effects of allergic phenotype on respiratory symptoms and exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:187–192.
-
Alcázar-Navarrete B, et al. Diagnostic performance of the measurement of nitric oxide in exhaled air in the diagnosis of COP phenotypes. NitricOxide.2016;54:67–72.
-
Fritzsching B, Zhou-Suckow Z, Trojanek JB, et al. Hypoxic epithelial necrosis triggers neutrophilic inflammation via IL-1 receptor signaling in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2015;191(8):902-913.
-
Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193(9):965-974.
-
Bélanger M, Couillard S, Courteau J, et al. Eosinophil counts in first COPD hospitalizations: a comparison of health service utilization. Int J Chron Obstruct Pulmon Dis. 2018;13:3045-3054.
-
George L, Taylor AR, Esteve- Codina A, et al; U-BIOPRED and the EvA study teams. Blood eosinophil count and airway epithelial transcriptome relationships in COPD versus asthma. Allergy. 2020;75(2):370-380
-
Yousuf A, Ibrahim W, Greening NJ, Brightling CE. T2 biologics for chronic obstructive pulmonary disease. J Allergy Clin Immunol Pract. 2019;7(5):1406-1416.
-
Barnes PJ. Inflammatory endotypes in COPD. Allergy. 2019;74(7):1249-1256.
-
Oishi K, Matsunaga K, Shirai T, Hirai K, Gon Y. Role of type 2 inflammatory biomarkers in chronic obstructive pulmonary disease. J Clin Med. 2020;9(8):2670. doi:10.3390/jcm9082670
-
Gabryelska A, Kuna P, Antczak A, Białasiewicz P, Panek M. IL-33 mediated inflammation in chronic respiratory diseases—understanding the role of the member of IL-1 superfamily. Front Immunol. 2019;10:692. doi:10.3389/fimmu.2019.00692
-
Allinne J, Scott G, Lim WK, et al. IL-33 blockade affects mediators of persistence and exacerbation in a model of chronic airway inflammation. J Allergy Clin Immunol. 2019;144(6):1624-1637.e10.
-
Calderon AA, Dimond C, Choy DF, et al. Targeting interleukin-33 and thymic stromal lymphopoietin pathways for novel pulmonary therapeutics in asthma and COPD. Eur Respir Rev. 2023;32(167):220144. doi:10.1183/16000617.0144-2022
-
Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos D. Targeting key proximal drivers of type 2 inflammation* in disease. Nat Rev Drug Discov. 2016;15(1):35-50.
-
Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119(6):1303-1310.
-
Doyle AD, Mukherjee M, LeSuer WE, et al. Eosinophil-derived IL-13 promotes emphysema. Eur Respir J. 2019;53(5):1801291. doi:10.1183/13993003.01291-2018
-
Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16-27.
-
Defrance T, Carayon P, Billian G, et al. Interleukin 13 is a B cell stimulating factor. J Exp Med. 1994;179(1):135-143.
-
Yanagihara Y, Ikizawa K, Kajiwara K, Koshio T, Basaki Y, Akiyama K. Functional significance of IL-4 receptor on B cells in IL-4– induced human IgE production. J Allergy Clin Immunol. 1995;96(6 pt 2):1145-1151.
-
Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425-437.
-
Kaur D, Hollins F, Woodman L, et al. Mast cells express IL-13Rα1: IL-13 promotes human lung mast cell proliferation and FcεRI expression. Allergy. 2006;61(9):1047-1053.
-
Saatian B, Rezaee F, Desando S, et al. Interleukin-4 and interleukin-13 cause barrier dysfunction in human epithelial cells. Tissue Barriers. 2013;1(2):e24333. doi:10.4161/tisb.24333
-
Zheng T, Zhu Z, Wang Z, et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase– and cathepsin-dependent emphysema. J Clin Invest. 2000;106(9):1081-1093.
-
Garudadri S, Woodruff PG. Targeting chronic obstructive pulmonary disease phenotypes, endotypes, and biomarkers. Ann Am Thorac Soc. 2018;15(suppl 4):S234-S238.
-
Alevy YG, Patel AC, Romero AG, et al. IL-13–induced airway mucus production is attenuated by MAPK13 inhibition. J Clin Invest. 2012;122(12):4555-4568.
-
Singanayagam A, Footitt J, Marczynski M, et al. Airway mucins promote immunopathology in virus-exacerbated chronic obstructive pulmonary disease. J Clin Invest. 2022;132(8):e12901. doi:10.1172/JCI120901
-
Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779-788.