UltraFlexi-1
It’s time to discover how Toujeo can help people with T1DM who exercise
UltraFlexi-1: The first RCT to compare Toujeo® and degludec 100 U/mL, in people with T1DM who exercise, using Time-below-Range as the primary endpoint.
The UltraFlexi-1 study
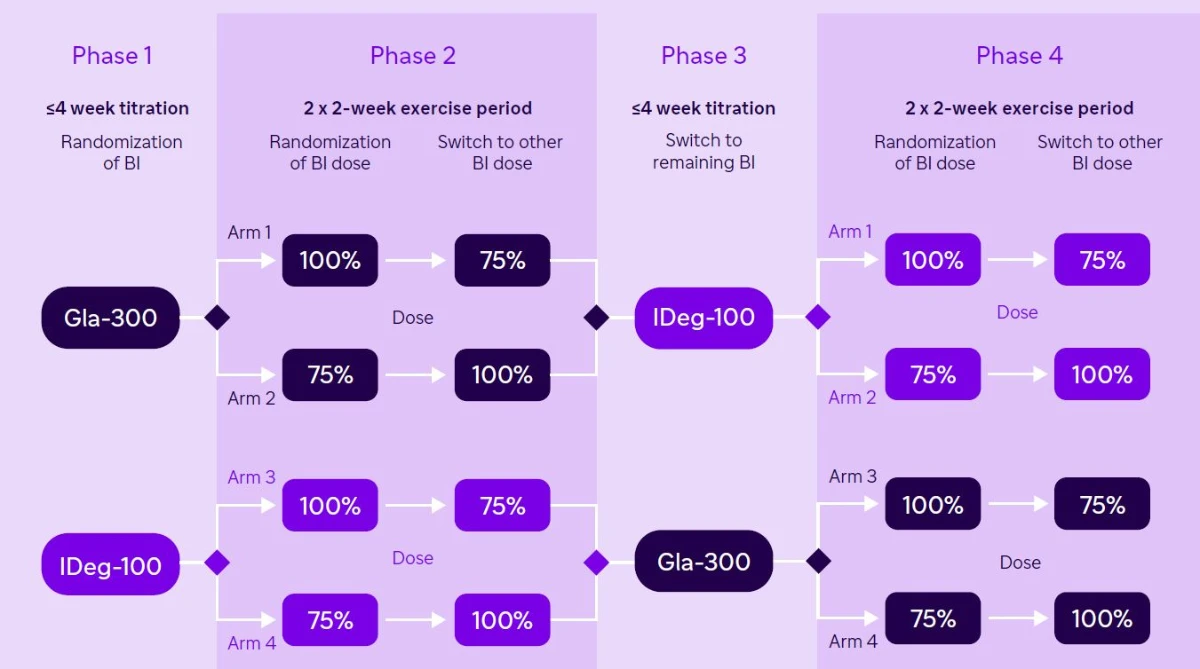
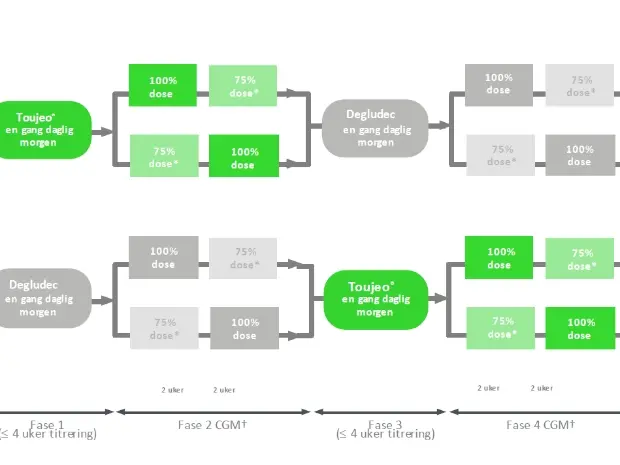
The randomized, single-center, crossover trial included adults with T1D treated with multiple daily insulin injections and an HbA1c of ≤10% (≤86 mmol/mol). Within each of the four 2-week periods, individuals attended 6 spontaneous 60-minute evening cycling sessions with moderate intensity. Each day of exercise was randomized and announced at 8 A.M. to the participant.
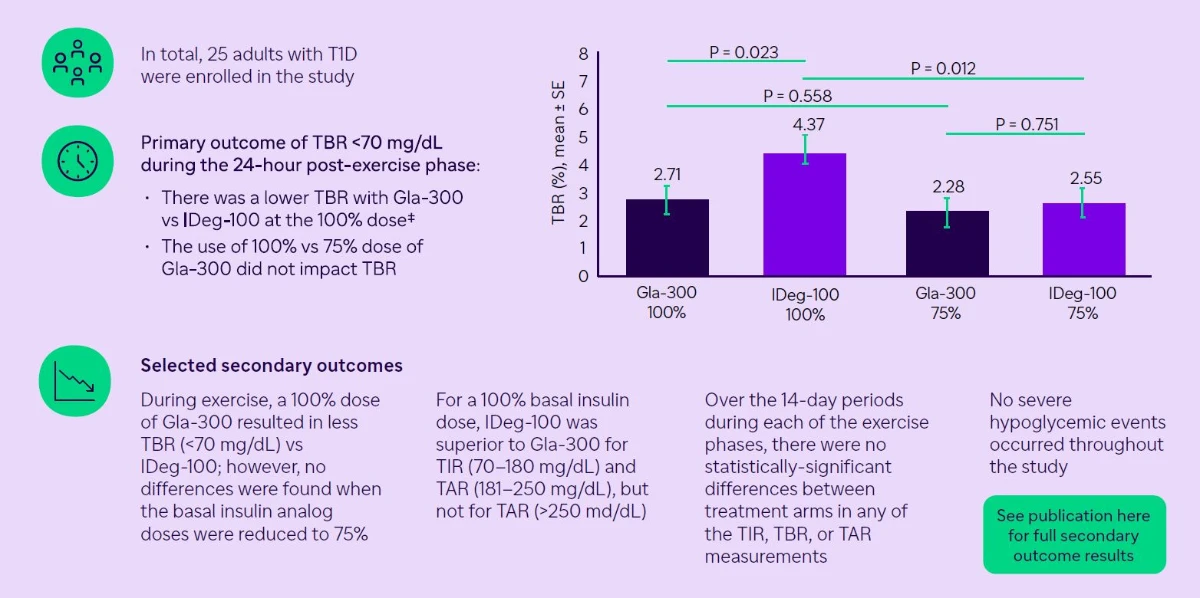
On non-exercise days, 100% of the regular basal insulin dose was used, while investigators performed CGM data analysis using a blinded Dexcom G6 device. The primary outcome was identified as the time below range (<70 mg/dL) during the six 24-hour post-exercise periods in the 4 trial arms. They analyzed the difference in time below the range between 100% IGlar and 100% IDeg or 75% IGlar and 75% IDeg in hierarchical order using the repeated measures linear mixed model.
A total of 25 individuals were enrolled in the study, including 14 male patients. They had a mean age of 41.4±11.9 years with a mean diabetes duration of 16.8 ± 10.4 years and a mean HbA1c of 7.5 ± 0.8%. (59±9 mmol/mol).
Conclusions:
- The fear of hypoglycemia or understanding how to manage hypoglycemia is a key barrier to exercise for people with T1D.
- Many people with T1D choose to split their dose of basal insulin, in order to provide more flexibility with insulin adjustments around exercise.
- The ULTRAFLEXI-1 study indicates that insulin dose adjustment of Gla-300 may not be necessary on days when spontaneous exercise is performed.
The study design
UltraFlexi-1 was a randomized, single-center, four-period, crossover trial, including adults with T1D treated with MDI insulin therapy and an HbA1c of <=10% (<=86 mmol/mol).
- During the four two week exercise intervention periods, participants wore a blinded rtCGM.
- Before the exercise period, participants consumed their usual breakfast with a regular prandial insulin dose.
- During the exercise period, it was recommended that participants consume a lunch containing at least 1 g·kg-1 CHO and a small snack with 15–30 g CHO both with a regular prandial insulin dose.
- After the exercise session, participants were asked to consume a dinner containing 1 g·kg-1 CHO with a regular or 25% reduced prandial insulin dose based on their preference.
Review by Dr. Othmar Moser, PhD
Listen to Dr. Othmar Moser, PhD from the Institute for Sports Science at the University of Bayreuth discussing the potential benefits and barrieres for exercise in people with type 1-diabetes and a detailed walkthrough of the UltraFlexi-1 study [8 min].
Type 1 diabetes og trening
- Moser O, et al Diabetes Technol Ther 2022;doi: 10 1089 /dia.2022.0422;
- https:://www.felleskatalogen.no/medisin/toujeo-sanofi-aventis-596100 (30.05.2023);
- https:://www.felleskatalogen.no/medisin/blaarev-register/a10ae04-1 (30.05.2023)
Toujeo - sanofi-aventis (Sanofi)
Human insulinanalog, langtidsvirkende. ATC nr.: A10A E04 (Insulin glargin)
Refusjonsberettiget bruk: Behandling av diabetes mellitus.
1. Indikasjoner: Behandling av diabetes mellitus hos voksne, ungdom og barn ≥6 år. 2. Dosering: Gis s.c. 1 gang daglig når som helst i løpet av døgnet, men til samme tid hver dag. 3 Kontraindikasjoner: Overfølsomhet for innholdsstoffene. 4 Vanligste bivirkninger: Hypoglykemier, reaksjoner på injeksjonsstedet og lipodystrofi. 5. Forsiktighetsregler: Skal ikke brukes til behandling av diabetisk ketoacidose. 6. Pakninger og listepriser: 300 enheter/ml: 3 stk × 1,5 ml (ferdigfylt penn, SoloStar) kr. 514,30. 5 stk x 1,5 ml (ferdigfylt penn, SoloStar) kr. 833,00. 300 enheter/ml: 3 stk. x 3ml (300 enheter/ml) kr. 992,30. 7. Refusjon og reseptgruppe3 : Blå resept og reseptgruppe C. Refusjon ytes kun til pasienter som ikke oppnår behandlingsmålene til tross for optimal behandling med middels langtidsvirkende NPH insulin på grunn av: - hyppige eller alvorlige nattlige føling er som skyldes insulinbruken - store blodsukkersvingninger som ikke gjør det mulig å oppnå akseptabel blodsukkerkontroll (180). Behandling skal kun startes av spesialist i indremedisin , barnesykdommer eller ved sykehusavdeling med tilsvarende spesialitet (181). Refusjon ytes kun til pasienter som til tross for optimal behandling med to daglige doser middels langtids vir kende NPH insulin har vedvarende utfordringer med hypoglykemier (244). 8. Refusjonskoder: ICPC - T89 (Diabetes type 1) og T90 (Diabetes type 2). ICD - E10 (Diabetes mellitus type 1) og E11 (Diabetes mellitus type 2).
Basert på SPC godkjent SLV/EMA: 20.11.2021. For mer informasjon se felleskatalogtekst eller SPC.

.webp/jcr:content.webp)
.webp/jcr:content.webp)