The Deliver-G study
DELIVER-G: Real-world analysis in insulin-naïve adults with T2DM on GLP-1 RA ± OAD(s), intensifying their therapy by starting basal insulin and discontinuing GLP-1 RA, or adding basal insulin to ongoing GLP-1 RA, or initiating a FRC product and discontinuing current GLP-1 RA†1
EASD and ADA recommend the addition of basal insulin for people with T2DM who do not achieve glycemic goals on a GLP-1 RA-based regimen.*2,3
The management of type 2 diabetes (T2D) often requires treatment intensification to achieve optimal glycemic control. The DELIVER-G study explores the real-world outcomes of adding Insulin Glargine 300 U/mL (Gla-300) to GLP-1 receptor agonist (GLP-1RA) therapy in people with type 2 diabetes (PWD2). This study builds on findings from the RESTORE-G real-world study, which examined treatment intensification following GLP-1RA therapy.
Observed in a real-world analysis in insulin-naïve adults with T2DM inadequately controlled on GLP-1 RA +/- OAD(s) (with ≥6 months of follow-up data)1*
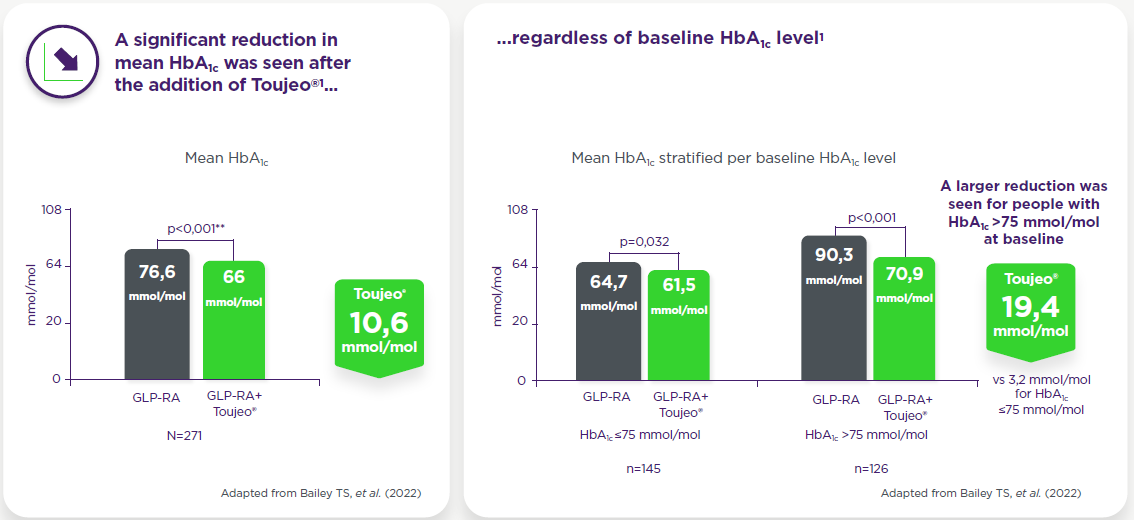
Observed in a real-world analysis in insulin-naïve adults with T2DM inadequately controlled on GLP-1 RA +/- OAD(s) (with ≥6 months of follow-up data)1*
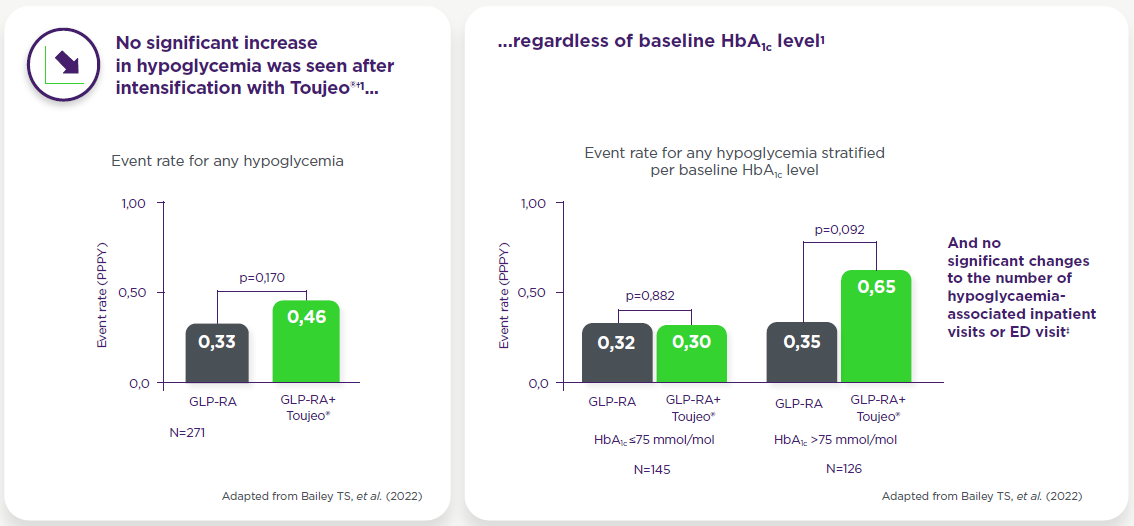
When analyzed by GLP-1RA regimen, increases in the incidence of hypoglycemia (from 3,9% at baseline to 8,3% at follow-up; p=0,020) were observed at follow-up in patients using daily GLP-1RA but no significant differences were seen in patients using weekly GLP-1RA.
Observed in a real-world analysis in insulin-naïve adults with T2DM inadequately controlled on GLP-1 RA +/- OAD(s) (with ≥6 months of follow-up data)1*
- No significant changes in weight (108,0 vs 108,4 kg) or BMI (35,8 vs 36,1 kg/m2)were seen with addition of Toujeo® to GLP-1RAs (baseline vs follow-up, respectively)
- However, a 3,9% increase in patients with BMI ≥35 kg/m2 was seen (51,5% vs 55,4%; p=0,046)
Hearing from diabetes experts
Study design
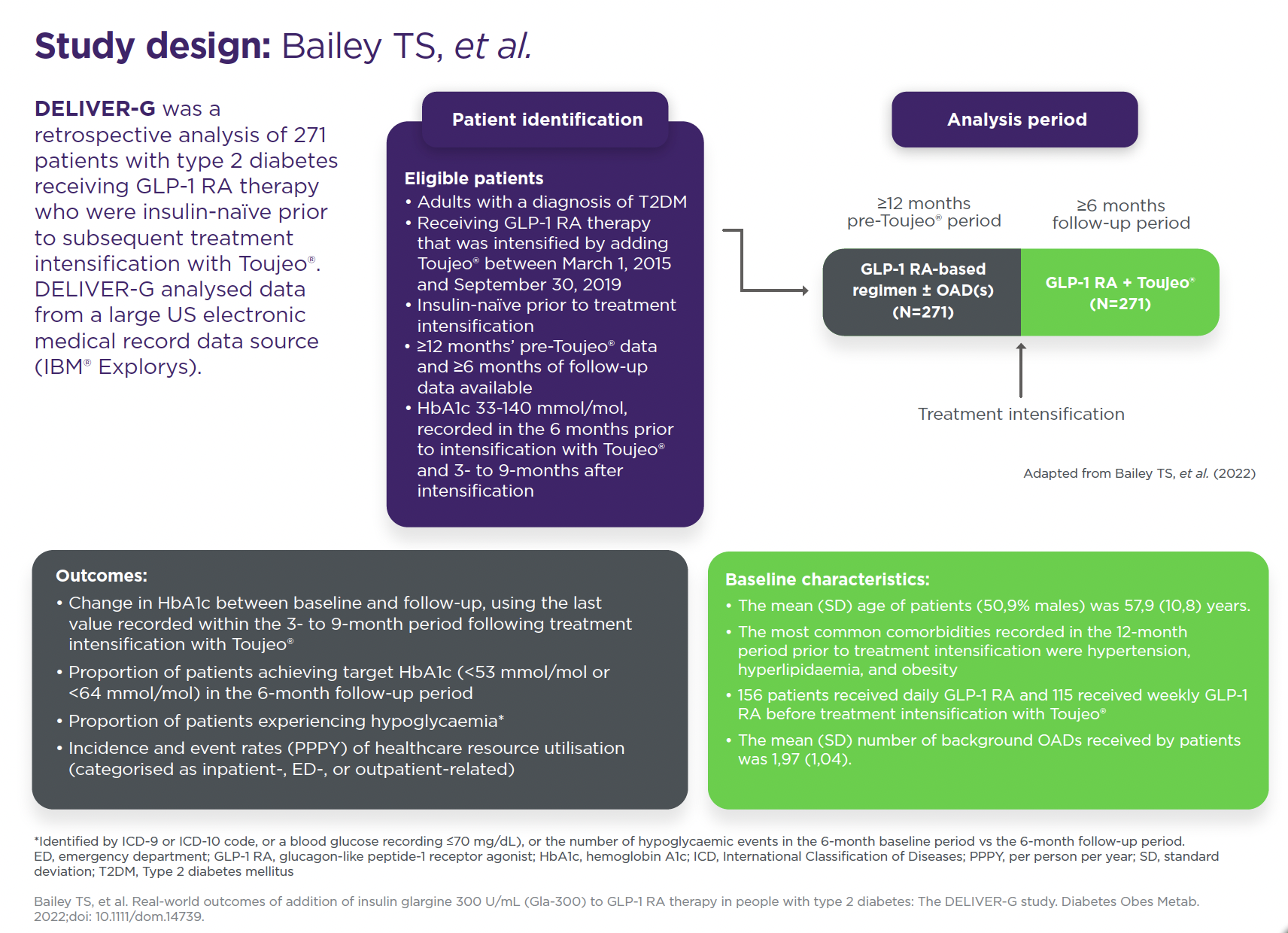
DELIVER-G was a retrospective analysis of 271 patients with type 2 diabetes receiving GLP-1 RA therapy who were insulin-naïve prior to subsequent treatment intensification with Toujeo®. DELIVER-G analysed data from a large US electronic medical record data source (IBM® Explorys).
Study population
Insulin-naïve adult patients (N=271) with T2DM receiving GLP-1 RA therapy ± OAD(s) that was subsequently intensified by adding Toujeo®. A total of 156 (57,6%) patients received daily GLP-1 RA and 115 (42,2%) received weekly GLP-1 RA before treatment intensification; 250 (92,3%) patients were receiving at least one OAD prior to intensification with Toujeo® (the mean number of OADs was two).
*Data were extracted from a US electronic medical record data source (IBM® Explorys). Patients selected for inclusion had ≥12 months’ pre-Toujeo® data and ≥6 months of follow-up data.
**Paired Student’s t-test. Change in HbA1c between baseline and follow-up was assessed using the last value recorded within the 3- to 9-month period following intensification with Toujeo®.
†Identified by ICD-9 or ICD-10 codes, or a blood glucose recording ≤70 mmol/L, or the number of hypoglycaemic events in the 6-month baseline period vs the 6-month follow-up period. Incidence of any hypoglycaemia pre- and post-intensification with Toujeo® was 8,49% and 9,59%, respectively (p=0,513). Paired Student’s t-test.
‡One hypoglycaemia-associated inpatient visit was recorded during the baseline period and none during the follow-up period after intensification with Toujeo®; no hypoglycaemia-associated ED visits were recorded during the baseline period and one during the follow-up period. Incidence of any (8,49% vs 9,59%, p=0,513), and inpatient/ED-associated hypoglycaemia (0,37% vs 0,74%, p=1,000), as well as event rates of any (0,33 vs 0,46 PPPY, p=0,170) and inpatient/ED-associated hypoglycaemia (0,01 vs 0,04 PPPY, p=0,466) were similar before and after addition of Toujeo®. When analysed by baseline GLP-1 RA regimen, incidence of hypoglycaemia increased from baseline to follow-up (3,9% to 8,3%, p=0,02; event rate 0,21 to 0,44 PPPY, p=0,017) for those on daily GLP-1RAs. No significant change was observed for those on weekly GLP-1 RAs.
- Bailey TS, et al. Real-world outcomes of addition of insulin glargine 300 U/mL (Gla-300) to GLP-1 RA therapy in people with type 2 diabetes: The DELIVER-G study. Diabetes Obes Metab. 2022;doi: 10.1111/dom.14739.
- Davies MJ, et al. Diabetes Care. 2018;41:2669–701;
- American Diabetes Association. Diabetes Care. 2024;47 (Suppl 1): S158-S178;
TOUJEO® (insulin glargine 300 units/ml) – Abbreviated Prescribing Information
NAME AND PRESENTATION: Toujeo 300 units/ml SoloStar, solution for injection in a prefilled pen. Toujeo 300 units/ml DoubleStar, solution for injection in a pre-filled pen. 1 ml of solution contains 300 units of insulin glargine. Each SoloStar prefilled pen contains 1,5 ml of solution for injection (equivalent to 450 units). Each DoubleStar pen contains 3 ml of solution for injection (equivalent to 900 units). THERAPEUTIC INDICATIONS: Treatment of diabetes mellitus in adults, adolescents and children from the age of 6 years. POSOLOGY AND METHOD OF ADMINISTRATION*: Toujeo is a basal insulin for once-daily administration at any time of the day, preferably at the same time every day. When needed, patients can administer Toujeo up to 3 hours before or after their usually time of administration. The dose regimen (dose and timing) should be adjusted according to individual response. In type 1 diabetes mellitus, Toujeo is to be used once-daily and must be combined with short-/rapid-action insulin to cover mealtime insulin requirements. In patients with type 2 diabetes mellitus, the recommended daily starting dose is 0.2 units/kg. Toujeo can also be given together with other anti-hyperglycaemic medicinal products. Switch: When switching from insulin glargine 100 units/ml to Toujeo, this can be done on a unit-to-unit basis. When switching from a treatment regimen with an intermediate or long-action insulin to a regimen with Toujeo, a change of the dose of the basal insulin may be required and the concomitant anti-hyperglycaemic treatment may need to be adjusted. Close metabolic monitoring is recommended during the switch and in the initial weeks thereafter. For switch details see full SmPC. Special populations: Toujeo can be used in elderly people, renal and hepatic impaired patients and adolescents and children ≥6 years. Renal impairment & hepatic impairment: insulin requirements may be diminished. Elderly: progressive deterioration of a renal function may lead to a steady decrease in insulin requirements. Children: Toujeo can be used in in adolescents and children based on the same principles as adult patients. When switching to Toujeo, dose reduction on basal and bolus insulins needs to be considered on an individual basis to minimize risk of hypoglycaemia. Safety and efficacy in children below 6 years have not been established, no data are available. Method of administration: For subcutaneous use only. Rotate injection sites to reduce the risk of lipodystrophy and cutaneous amyloidosis. Toujeo must not be administered intravenously or in insulin infusion pumps. The Toujeo SoloStar and Toujeo DoubleStar pre-filled pens have been specifically designed for Toujeo and no dose re-calculation is required for either pen. When changing from Toujeo SoloStar to Toujeo DoubleStar, if the patient’s previous dose was an odd number (e.g. 23 units) then the dose must be increased or decreased by 1 unit (e.g. 24 or 22 units). Toujeo DoubleStar prefilled pen is recommended for patients requiring at least 20 units per day. Toujeo must not be drawn from the cartridge of the Toujeo SoloStar pre-filled pen or Toujeo DoubleStar pre-filled pen into a syringe or severe overdose can result. For administration details see full SmPC. CONTRAINDICATIONS: Hypersensitivity to the active substance or to any of the excipients listed in the full SmPC. SPECIAL WARNINGS AND PRECAUTIONS FOR USE*: Record name and batch number of administered product to improve traceability of biological medicinal products. Toujeo is not the insulin of choice for the treatment of diabetic ketoacidosis. Instruct patients to continuously rotate injection site to reduce risk of lipodystrophy and cutaneous amyloidosis; delayed insulin absorption and worsened glycaemic control may occur after injection at affected sites. Sudden change in injection site to unaffected area has resulted in hyperglycaemica; blood glucose monitoring is recommended after changing injection site and dose adjustment of antidiabetic medications may be considered. The prolonged effect of insulin glargine may delay recovery from hypoglycaemia. Insulin glargine 100 units/ml and Toujeo are not bioequivalent and are not interchangeable and switching may result in the need for a change in dose and should only be done under strict medical supervision. Switching patients between other insulins and Toujeo should be done under strict medical supervision and may result in the need for a change in dose. Intercurrent illness requires intensified metabolic monitoring. In rare cases the presence of insulin antibodies may necessitate adjustment of insulin dose. If pioglitazone is used in combination with insulin, especially in patients with risk factors for development of cardiac heart failure, patients should be observed for signs and symptoms of heart failure, weight gain and oedema. Pioglitazone should be discontinued if any deterioration in cardiac symptoms occurs. For further details on special warnings and precautions for use see full SmPC. DRUG INTERACTIONS*: Substances that may enhance or reduce the blood glucose-lowering activity and increase susceptibility to hypoglycaemia are detailed in the full SmPC. PREGNANCY AND LACTATION*: There is no clinical experience with use of Toujeo in pregnant women. For insulin glargine no clinical data on exposed pregnancies from controlled clinical studies are available. A large amount of data on pregnant women indicate no specific adverse effects on pregnancy and no specific malformative nor feto/neonatal toxicity of insulin glargine. The use of Toujeo may be considered during pregnancy if clinically needed. It is unknown whether insulin glargine is excreted in human milk. No metabolic effects on breast-fed newborn/infant are anticipated as insulin glargine is digested into amino acids in the gastrointestinal tract. EFFECTS ON ABILITY TO DRIVE*: Patient’s ability to concentrate and react may be impaired as a result of hypoglycaemia or hyperglycaemia or, for example, as a result of visual impairment. Patients should be advised to take precautions to avoid hypoglycaemia whilst driving. UNDESIRABLE EFFECTS*: Very common: Hypoglycemia. Hypoglycaemia may occur if the insulin dose is too high in relation to the insulin requirement. Common: Lipohypertrophy; injection site reactions. For full list of undesirable effects consult the full SmPC. Clinical study safety data not available for children <6 years. OVERDOSAGE*: Mild episodes of hypoglycaemia can usually be treated with oral carbohydrates. More severe episodes may be treated with intramuscular/subcutaneous glucagon or concentrated intravenous glucose. PHARMACOLOGICAL PROPERTIES: ATC Code: A10A E04. MARKETING AUTHORIZATION HOLDER: Sanofi-Aventis Deutschland GmbH, D 65926 Frankfurt am Main, Germany. LEGAL CATEGORY: Medicinal product subject to medical prescription. DATE OF LAST REVIEW: Aug 2024
Abbreviated Prescribing information based on the EU SMPC as of Nov 2023.
Before prescribing the product always refer to your full local prescribing information as this information may vey from country to country.
For local details, please refer to below information.
Sweden: Rx, (F), A10AE04. Toujeo is reimbursed for all patients with type 1-diabetes and for patients with type 2-diabetes where other insulin treatments are not sufficient to reach the treatment target because of repeated hypoglycaemic events. For further information, and also information concerning price and packaging, see www.fass.se. In Sweden Toujeo is provided by Sanofi AB, Box 30052, 104 25 Stockholm, Tel: +46 8 634 50 00, www.sanofi.se. For questions on our medicinal products, please contact: infoavd@sanofi.com.