Nexviadyme®
A treatment for patients with Pompe disease1
Nexviadyme® (avalglucosidase alfa) is an enzyme replacement monotherapy (ERT) indicated for the long-term treatment of patients with Pompe disease (acid α-glucosidase deficiency), both infantile form (IOPD) and late form (LOPD).1
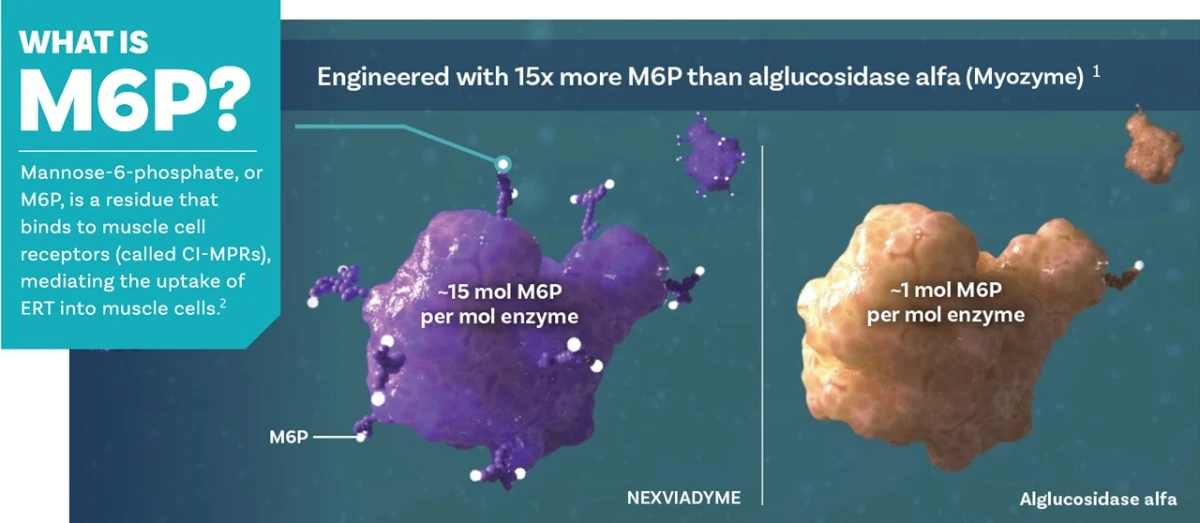
The mechanism of action is similar to that of Myozyme (alglucosidase alfa), but its structure is enriched with M6P (mannose-6-phosphate), for better cellular uptake and better breakdown of glycogen.2
How Nexviadyme® works 1,2
Nexviadyme
Dosing1
Nexviadyme® The recommended dose of avalglucosidase alfa is 20 mg/kg of body weight administered once every 2 weeks.
For IOPD patients who experience lack of improvement or insufficient response in cardiac, respiratory, and/or motor function while receiving 20 mg/kg, a dose increase to 40 mg/kg every other week should be considered in the absence of safety concerns (e.g., severe hypersensitivity, anaphylactic reactions, or risk of fluid overload).
Monotherapy administered bi-weekly via intravenous infusion, supervised by an experienced physician in the management of Pompe disease.
Each pack contains 1 single-use vial, containing 100 mg of avalglucosidase alfa
After reconstitution, each vial contains a total extractable volume of 10.0 ml at a concentration of 10 mg of avalglucosidase alfa per ml.
Compared to Myozyme, Nexviadyme® has twice the medication per vial, which reduces the number of vials.1,3
Reconstitution and administration of Nexviadyme®1
Watch the video below to learn more about how to reconstitute and administer Nexviadyme®.
Nexviadyme
The safety profile - related to hypersensitivity (including anaphylaxis) and infusion-associated reactions (IARs)1
In a pooled safety analysis, hypersensitivity reactions were reported in 60.6% of patients, anaphylaxis in 2.8%, and IARs in 39.4% of patients.
Please see the complete safety profile for Nexviadyme in the SmPC.
Summary of Product Characteristics - Nexviadyme® (avalglucosidase alfa)
Click the link below and get access to the Summary of Product Characteristics for more information on Nexviadyme®.
Summary of Product Characteristics - Myozyme® (alglucosidase alfa)
Click the link below and get access to the Summary of Product Characteristics for more information on Myozyme®.
References
-
Nexviadyme Summary of Product Characteristics. April 2024
-
Zhu Y, Jiang JL, Gumlaw NK et al. Glycoengineered acid alpha-glucosidase with improved efficacy at correcting the metabolic aberrations and motor function deficits in a mouse model of Pompe disease. Mol Ther. 2009 Jun; 17(6): 954–963.
-
Myozyme Summary of Product Characteristics. March 2024
MAT-SE-2400593 v1.0 08/2024