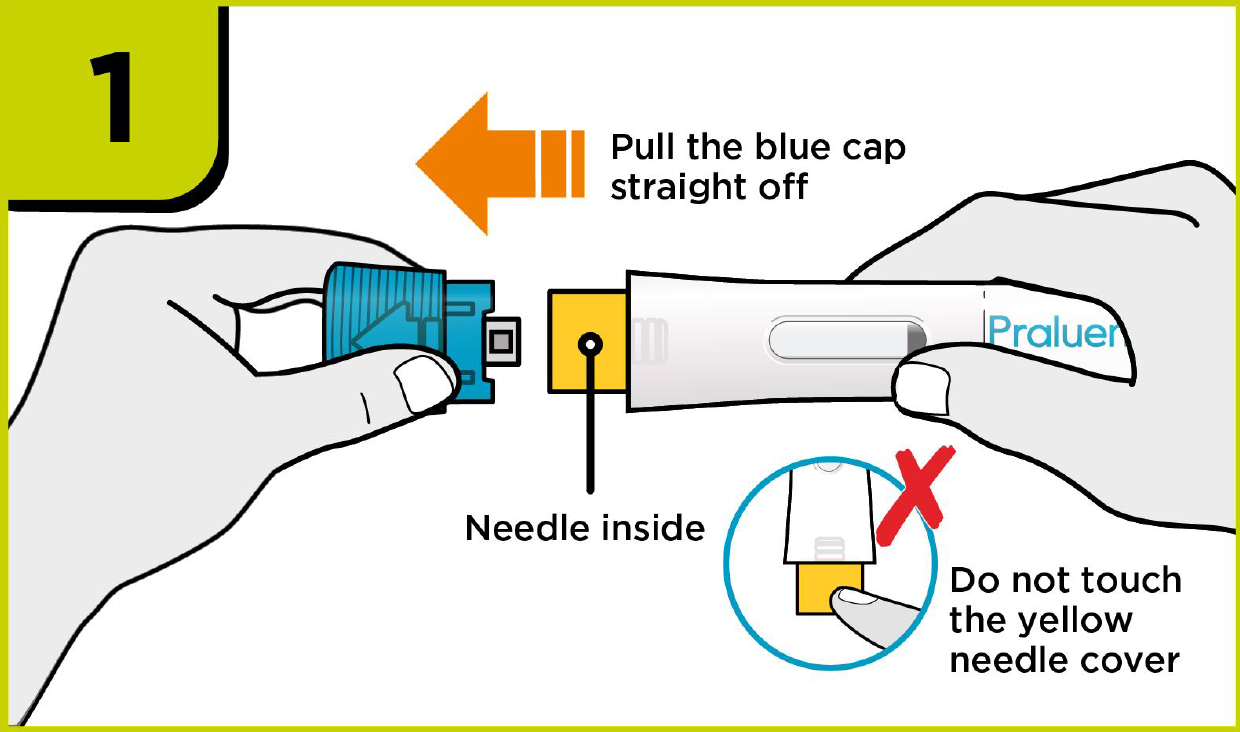
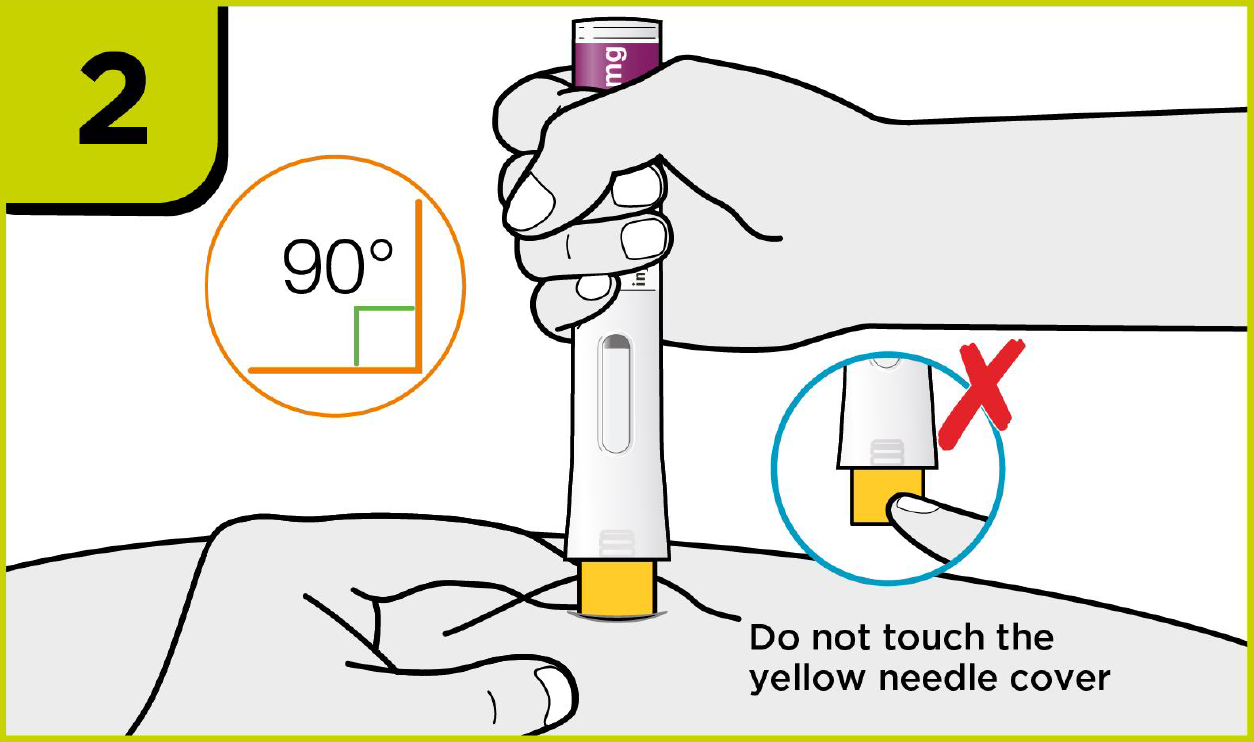
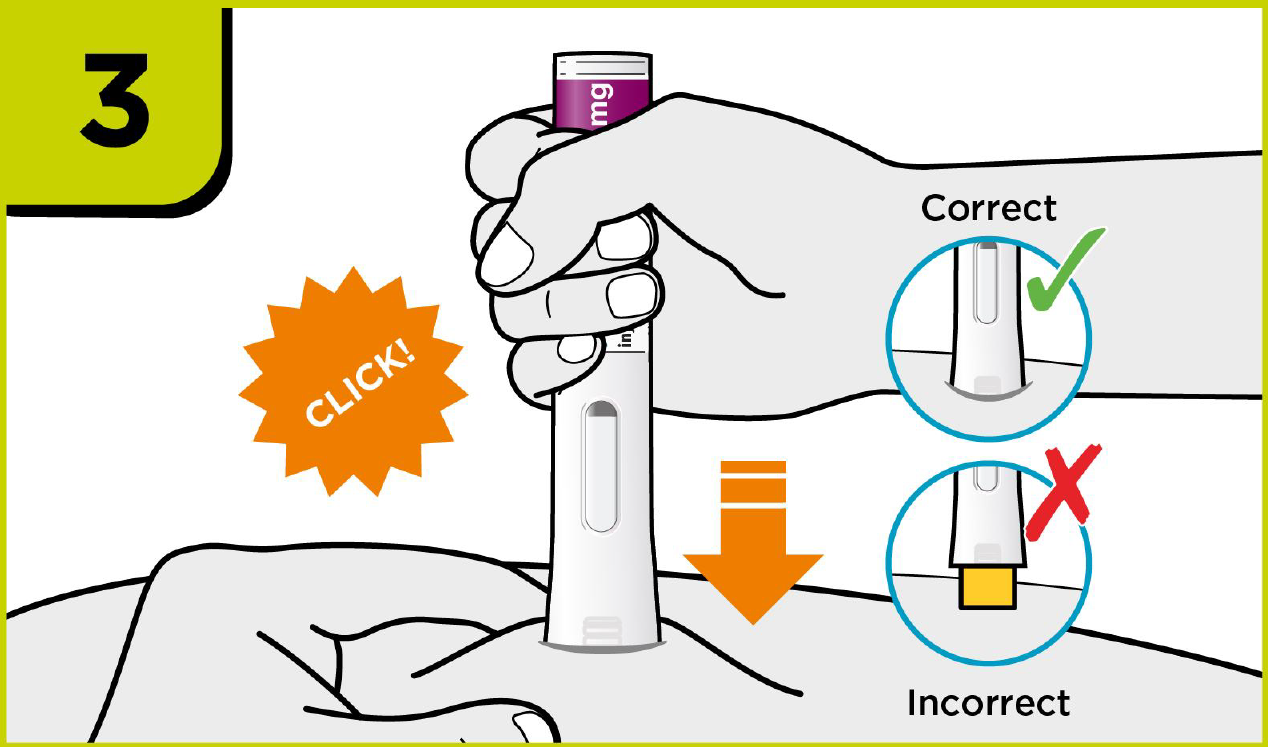
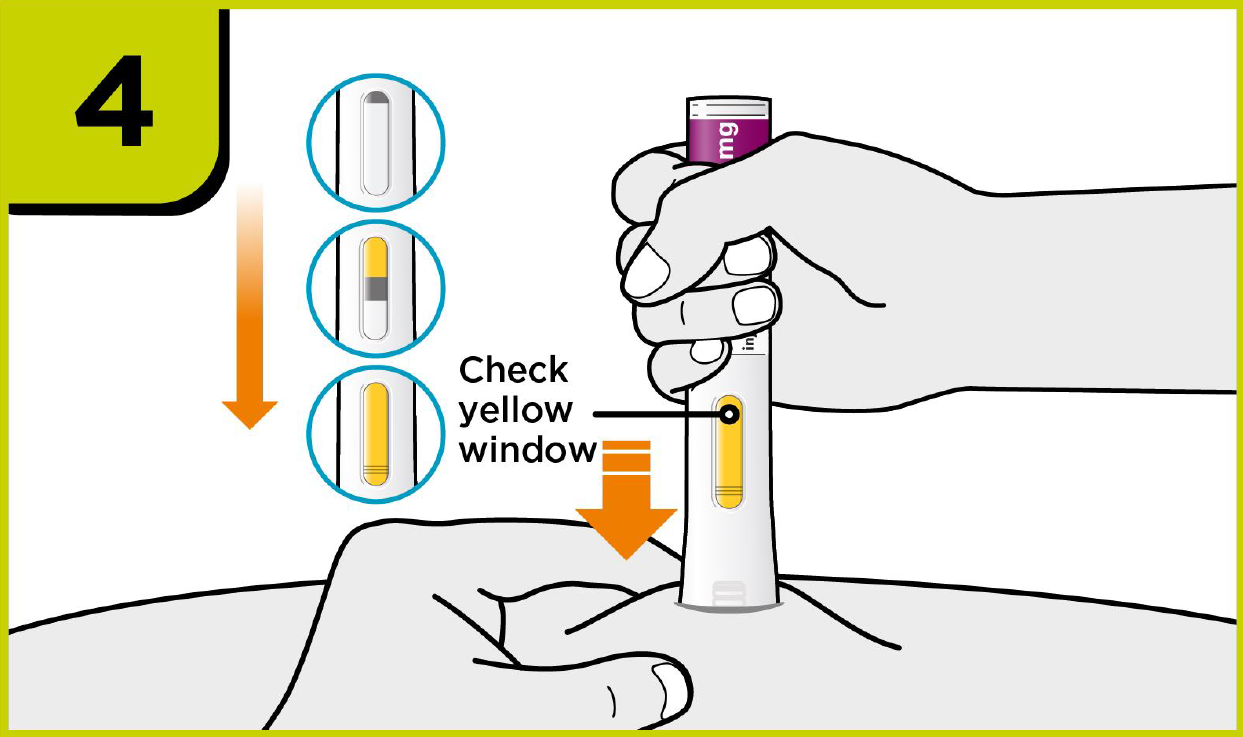
How to use PRALUENT®?
PRALUENT is available as a unique,¥ once-monthly pen for your patients requiring >60% LDL-C reduction2,3
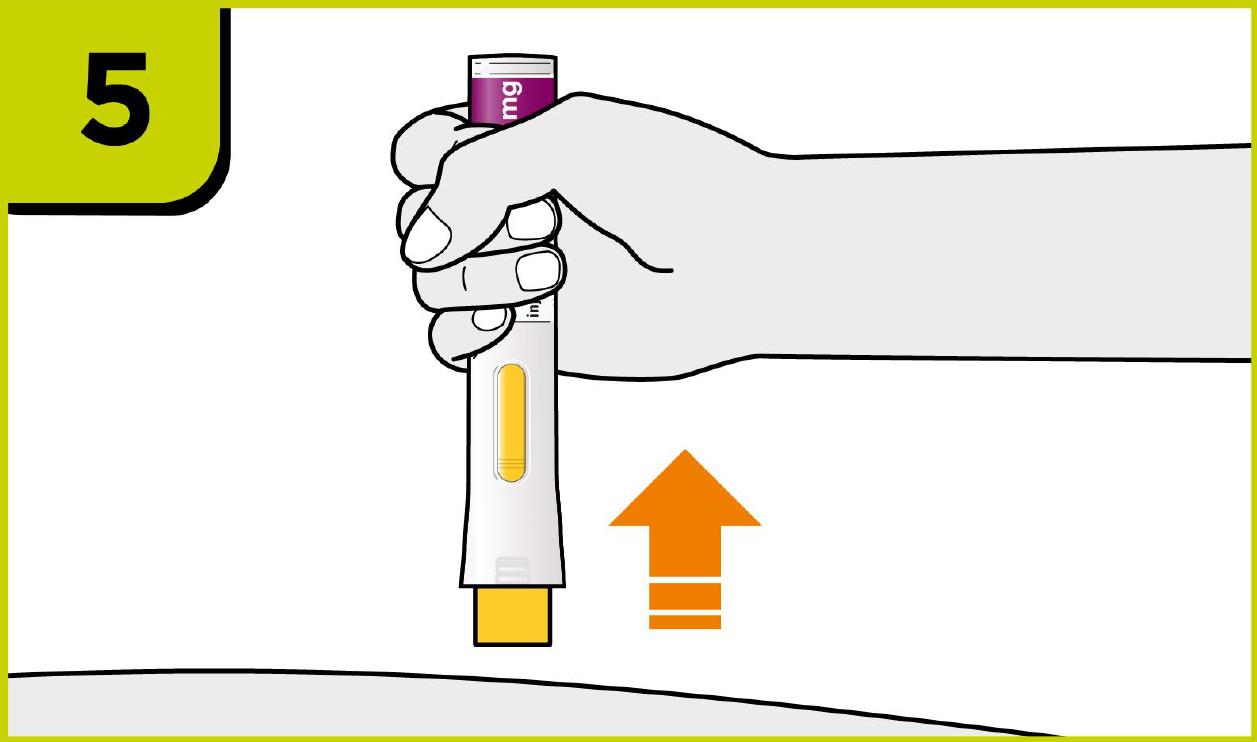
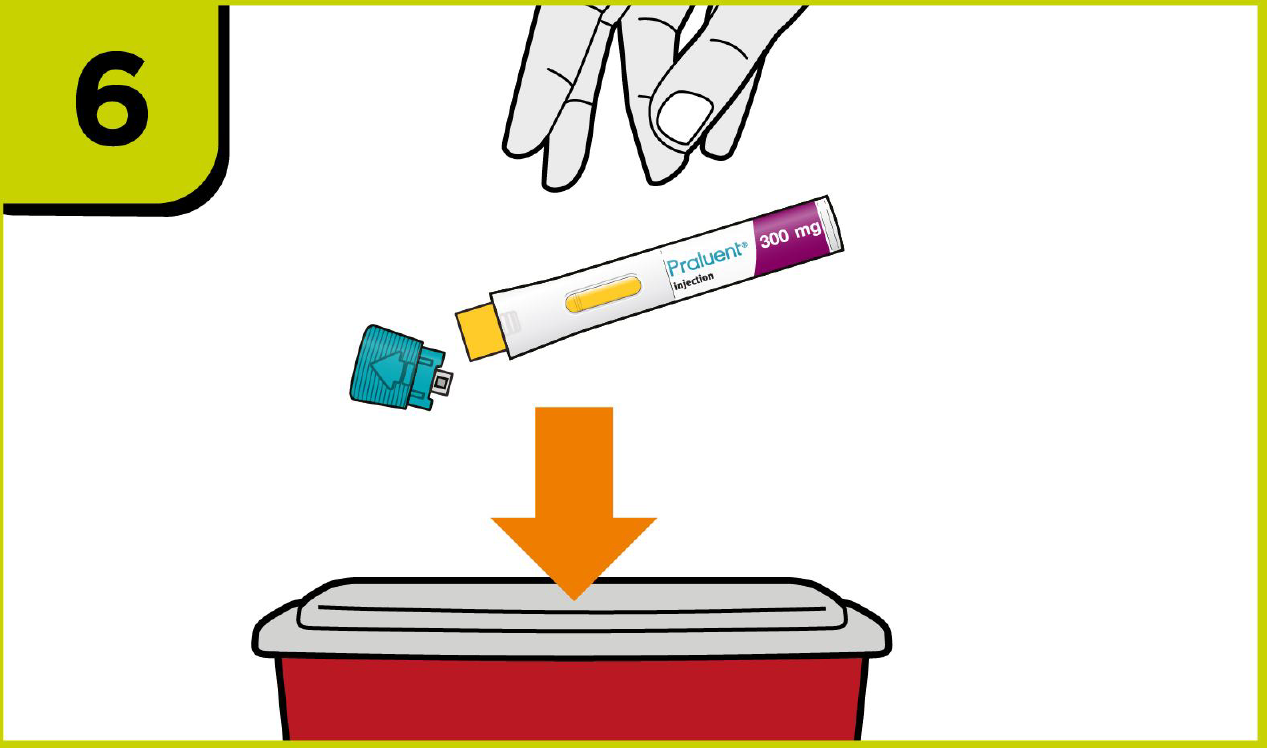
Self-injection summary2
Regardless of their dose, patients will now follow the same injection steps with the new device, which could help to streamline day-to-day management for you.2
More information
Click a link below to learn more about PRALUENT
What is PRALUENT?
Click here to learn about how PRALUENT works and who it is for
Why choose PRALUENT?
Click here to learn more about the efficacy and safety profile of PRALUENT
Indication, Dosing, and Footnotes
NAME OF THE MEDICINAL PRODUCT: Praluent 75 mg, 150 mg or 300 mg solution for injection in pre-filled pen. QUALITATIVE AND QUANTITATIVE COMPOSITION: Each single-use pre-filled pen contains 75 mg alirocumab in 1 ml solution, 150 mg alirocumab in 1 ml solution or 300 mg in 2 ml solution. THERAPEUTIC INDICATIONS: Primary hypercholesterolaemia and mixed dyslipidaemia Praluent is indicated in adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia and in paediatric patients 8 years of age and older with heterozygous familial hypercholesterolaemia (HeFH) as an adjunct to diet: - in combination with a statin or statin with other lipid lowering therapies in patients unable to reach LDL-C goals with the maximum tolerated dose of a statin or,- alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated. Established atherosclerotic cardiovascular disease Praluent is indicated in adults with established atherosclerotic cardiovascular disease to reduce cardiovascular risk by lowering LDL-C levels, as an adjunct to correction of other risk factors: - in combination with the maximum tolerated dose of a statin with or without other lipid-lowering therapies or, - alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated. POSOLOGY AND METHOD OF ADMINISTRATION: Prior to initiating alirocumab secondary causes of hyperlipidaemia or mixed dyslipidaemia (e.g., nephrotic syndrome, hypothyroidism) should be excluded. The usual starting dose for alirocumab is 75 mg administered subcutaneously once every 2 weeks. Patients requiring larger LDL-C reduction (>60%) may be started on 150 mg once every 2 weeks, or 300 mg once every 4 weeks (monthly), administered subcutaneously. The dose of alirocumab can be individualised based on patient characteristics such as baseline LDL-C level, goal of therapy, and response. Lipid levels can be assessed 4 to 8 weeks after treatment initiation or titration, and dose adjusted accordingly (up-titration or down-titration). If additional LDL-C reduction is needed in patients treated with 75 mg once every 2 weeks or 300 mg once every 4 weeks (monthly), the dosage may be adjusted to the maximum dosage of 150 mg once every 2 weeks. If a dose is missed, the patient should administer the injection as soon as possible and thereafter resume treatment on the original schedule. No dose adjustment is needed for elderly patients, or patients with mild or moderate hepatic or renal impairment, in patients based on weight. Alirocumab has not been studied in paediatric patients less than 8 years of age. Method of administration Subcutaneous injection into the thigh, abdomen or upper arm. Each pre-filled pen is for single use only. To administer the 300 mg dose, either one 300 mg injection or two 150 mg injections should be given consecutively at two different injection sites. It is recommended to rotate the injection site with each injection. Alirocumab should not be injected into areas of active skin disease or injury such as sunburns, skin rashes, inflammation, or skin infections. Alirocumab must not be co-administered with other injectable medicinal products at the same injection site. The patient may either self-inject alirocumab, or a caregiver may administer alirocumab, after guidance has been provided by a healthcare professional on proper subcutaneous injection technique. The solution should be allowed to warm to room temperature prior to use. CONTRAINDICATIONS: Hypersensitivity to the active substance or to any of the excipients. SPECIAL WARNINGS AND PRECAUTIONS FOR USE: Traceability In order to improve the traceability of biological medicinal products, the name and the batch number of the administered product should be clearly recorded. Allergic reactions General allergic reactions, including pruritus, as well as rare and sometimes serious allergic reactions such as hypersensitivity, nummular eczema, urticaria, and hypersensitivity vasculitis have been reported in clinical studies. Angioedema has been reported in the postmarketing setting. If signs or symptoms of serious allergic reactions occur, treatment with alirocumab must be discontinued and appropriate symptomatic treatment initiated. Renal impairment In clinical studies, there was limited representation of patients with severe renal impairment (defined as eGFR < 30 ml/min/1.73 m2). Alirocumab should be used with caution in patients with severe renal impairment. Hepatic impairment Patients with severe hepatic impairment (Child-Pugh C) have not been studied. Alirocumab should be used with caution in patients with severe hepatic impairment. UNDESIRABLE EFFECTS: injection site reactions, upper respiratory tract signs and symptoms, pruritus, urticaria, eczema nummular hypersensitivity. MARKETING AUTHORISATION HOLDER: Sanofi Winthrop Industrie 54, rue La Boétie F-75008 Paris, France. DATE OF REVISION OF THE TEXT: Nov 2024. Medicinal product subject to restricted medical prescription. Detailed information on this medicine is available on European Medicines Agency website http://www.ema.europa.eu.
PRESCRIPTION MEDICATION. ATC-code: C10AX14.Reimbursement: Reimbursed for patients with diagnosed heterozygous familial hypercholesterolemia who, despite maximum tolerable treatment with statin and ezetimibe, have residual LDL cholesterol of 2.6 mmol/L or higher. Reimbursed for patients with diagnosed atherosclerotic cardiovascular disease who, despite maximum tolerable treatment with statin and ezetimibe, have residual LDL cholesterol of 1.8 mmol/L or higher. Reimbursed for patients with diagnosed diabetes mellitus and target organ damage (microalbuminuria, retinopathy, or neuropathy), or at least three major risk factors, or early onset of type 1 diabetes mellitus with long duration, who, despite maximum tolerable treatment with statin and ezetimibe, have residual LDL cholesterol of 2.6 mmol/L or higher. For more information see fass.se(https://www.fass.se/LIF/product?userType=0&nplId=20141231000088). Sanofi AB. Phone +46-8-634 50 00.
Adults2
Prior to initiating PRALUENT® secondary causes of hyperlipidaemia or mixed dyslipidaemia (e.g., nephrotic syndrome, hypothyroidism) should be excluded.
The usual starting dose for alirocumab is 75 mg administered subcutaneously once every 2 weeks. Patients requiring larger LDL-C reduction (>60%) may be started on 150 mg once every 2 weeks, or 300 mg once every 4 weeks (monthly), administered subcutaneously.
The dose of PRALUENT® can be individualised based on patient characteristics such as baseline LDL-C level, goal of therapy, and response. Lipid levels can be assessed 4 to 8 weeks after treatment initiation or titration, and dose adjusted accordingly (up-titration or down-titration).
If additional LDL-C reduction is needed in patients treated with 75 mg once every 2 weeks or 300 mg once every 4 weeks (monthly), the dosage may be adjusted to the maximum dosage of 150 mg once every 2 weeks.
HeFH in paediatric patients 8 years of age and older:2
| Body weight of patients | Recommended dose | Recommended dose if additional LDL-C reduction is needed* |
| Less than 50 kg | 150 mg once every 4 weeks | 75 mg once every 2 weeks |
| 50 kg or more | 300 mg once every 4 weeks | 150 mg once every 2 weeks |
Adapted from PRALUENT® Summary of Product Characteristics. 2024.2
* Lipid levels can be assessed 8 weeks after treatment intiation or titration and dose adjusted accordingly.2
If a dose is missed, the dose should be administered as soon as possible and thereafter, dosing should be resumed on the original schedule.
*62.7% LDL-C reduction compared to placebo at 4 months in ODYSSEY OUTCOMES trial.1
†MACE: primary composite endpoint of CHD death, non-fatal myocardial infarction, fatal and non-fatal ischaemic stroke, or unstable angina requiring hospitalisation. HR 0.85 (95% CI 0.78, 0.93), P=0.0003.1,2
‡With only nominal statistical significance by hierarchical testing; HR 0.85 (95% Cl 0.73, 0.98), P=0.0261.1,2
¥For patients requiring LDL-C reduction >60%, PRALUENT is the only PCSK9i with once-monthly single injection in a pre-filled pen.2
CI = confidence interval; HeFH = Heterozygous Familial Hypercholesterolaemia; HR = hazard ratio; LDL-C = low-density lipoprotein cholesterol; MACE = major adverse cardiovascular event; PCSK9i = proprotein convertase subtilisin/kexin type inhibitor.
-
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107.
-
PRALUENT (alirocumab) Summary of Product Characteristics. Paris, France: sanofi-aventis groupe; 10/12/2024
-
Frias JP, Koren MJ, Loizeau V, et al. The SYDNEY device study: a multicenter, randomized open-label usability study of a 2-mL alirocumab autoinjector device. Clin Ther. 2020;42(1):94–107.