Understanding Disease Modification in Atopic Dermatitis
Disease modification in atopic dermatitis (AD) represents a paradigm shift from symptom control to targeting the underlying pathomechanisms. According to a recent publication, disease modification in AD could be demonstrated by reversing the underlying pathophysiology, achieving clinical and subclinical remission, and preventing the development of comorbidities.1
Concepts of disease modification in AD
|
|
Sustained on-therapy remission: “Any intervention that endurably impacts the pathomechanisms and the natural course of the disease, leading to sustained remission”1 |
|
|
Improvement or prevention of comorbidities: “An intervention successfully preventing the development or the progression of atopic comorbidities (e.g. food allergy, allergic asthma and/or allergic rhinitis), before or during their development”1 |

|
Sustained off-therapy remission: “A state where the patient remains in remission without the need for ongoing medical treatment”1 |

|
Sub-clinical remission: “Surrogate biomarkers provide insights on mechanisms leading to the associated comorbidities, have predictive value for short-, mid- and long-term responses to disease modifying therapies”1 |
1 - Achieving clinical remission (disease control)
Clinical remission in atopic dermatitis refers to the visible clearance of skin lesions and the resolution of symptoms.1 The impact on disease control can be assessed using various outcome measures (EASI, SCORAD, etc.). It can be evaluated by looking at the percentage of weeks reported as well-controlled weeks or by evaluating the annualized flare rate during the treatment period to evaluate flare prevention. Additional evaluation measures help measure disease control such as the Atopic Dermatitis Control Tool or ADCT, which can be used to identify patients with uncontrolled disease.2
Regular administration of dupilumab led to sustained disease control in clinical and real-world settings3-7
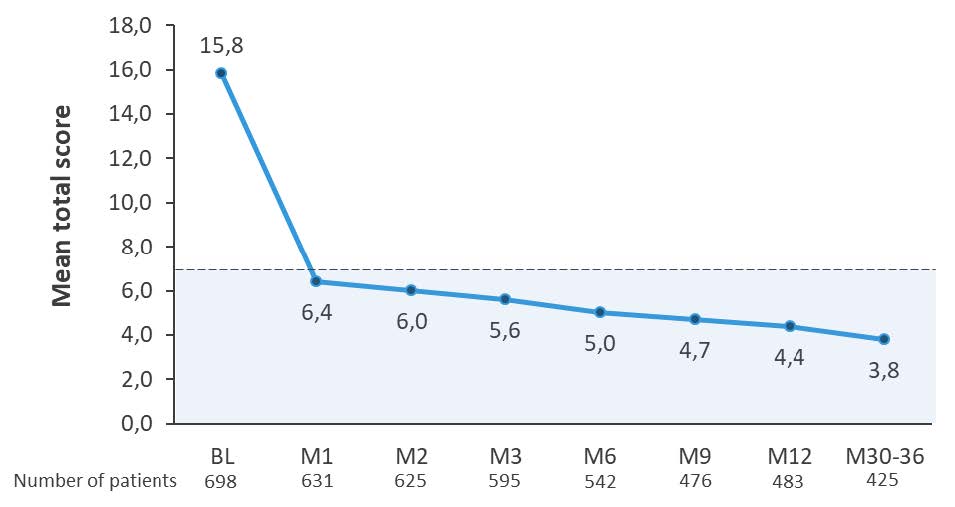
In clinical trials, dupilumab demonstrated sustained and significant improvements in disease control in patients with moderate-to- severe atopic dermatitis. Patients treated with dupilumab reported well-controlled disease during 88% of weeks compared with only 19% in the placebo group (LIBERTY AD CHRONOS study, 52-week phase 3 trial in moderate-to-severe AD).3 Furthermore, in the same study, annualized flare rates over 52 weeks showed dupilumab + TCS reduced flares 4.5-fold versus TCS alone (P < 0.0001).4 Long-term data from the adult open-label extension study showed that 88,9% of patients maintained at least a 75% improvement in disease severity over 5 years (LIBERTY ADULT OLE).5 In the real-world setting, results from the RELIEVE AD study further reinforced the durability of response, showing sustained disease control (ADCT score <7) as early as Month 1 and maintained up to 30-36 months (Figure 1: Early disease control (defined as total ADCT score <7a) from Month 1 sustained up to 30–36 Months, RELIEVE AD).6,7
Figure 1: Early disease control (defined as total ADCT score <7a) from Month 1 sustained up to 30–36 monthsb;6,7
The figure is drawn up by Sanofi based on reference 6, Table 2 and reference 7, Figure 3a
aThe ADCT assesses the impact of 6 AD symptoms over the previous week (ADCT total score 0–24). Vertical bars represent the range of imputed outcome values for the study follow-up period using pattern mixture models, for patients who completed the BL survey.b P<0.001 for all time points vs BL. AD, atopic dermatitis; ADCT, Atopic Dermatitis Control Tool; BL, baseline; M, month. PP-NRS, Peak Pruritus Numeric Rating Scale; q2w every 2 weeks; TCS, topical corticosteroids.
43% of adolescents and 60% of pediatric patients treated with dupilumab for at least 1 year maintained clear/almost clear skin while off therapy8,9
Among 29.4% of adolescent patients aged 12-17 years with moderate-to-severe AD treated for ≥52 weeks with dupilumab who had clear/almost clear skin sustained for 12 weeks ("sustained remission on therapy") and then stopped medication, 43.3% maintained clear skin off-therapy for a median follow-up period of 18 weeks (Figure 2, LIBERTY AD PED-OLE).8
Among 28,7% of children aged 6-11 years with severe AD treated for ≥52 weeks with dupilumab who had clear/almost clear skin sustained for 12 weeks ("sustained remission on therapy") and then stopped medication, 60.3% maintained clear skin off-therapy for a median follow-up period of 15.7 weeks (Figure 3, LIBERTY AD PED-OLE).9
Figure 2: LIBERTY PED-OLEa, in adolescent patients (aged 12–17 years) with moderate-to-severe AD treated for ≥52 weeks8,f
Figure 3: LIBERTY PED-OLEa, In children (aged 6–11 years) with severe AD treated for ≥52 weeks9,b
AD, atopic dermatitis; OLE, open-label extension.
aLIBERTY AD PED-OLE is an ongoing OLE study in patients aged ≥6 months to <18 years with moderate-to-severe AD who participated in dupilumab parent studies. bResults are presented for the cohort aged ≥6 to <12 years with severe AD, with a data cutoff date of July 1, 2020. Patients who had sustained remission of the disease as defined by the maintenance of an IGA score of 0 or 1 continuously for 12 weeks after Week 40 were discontinued from study drug (eg, a patient who has an IGA score of 0 or 1 through Week 40 to Week 52, inclusive, will be discontinued from the study drug at Week 52; similarly, a patient who has an IGA score of 0 or 1 through Week 52 to Week 64, inclusive, will be discontinued from study drug at Week 64, and so on). cDefined as maintenance of an IGA score of 0 or 1 (clear/almost clear) for ≥12 weeks after 40 weeks on dupilumab. dMaintained clear/almost clear skin for at least 12 weeks. ePatients who reached an IGA score of ≥2 (mild disease and above) within the follow-up period re-started treatment with dupilumab. In these cases, investigators were encouraged to consider treatment with topical therapy and to re-initiated dupilumab only for patients who did not respond adequately after 7 days of topical treatment. fResults are presented for the cohort aged ≥12 to <18 years from the study with a data cutoff date of December 15, 2018. Patients who had sustained remission of the disease as defined by maintenance of an IGA score of 0 or 1 continuously for a 12-week period after Week 40 were discontinued from study drug (eg, a patient who has an IGA score of 0 or 1 through Week 40 to Week 52, inclusive, will be discontinued from the study drug at Week 52; similarly, a patient who has an IGA score of 0 or 1 through Week 52 to Week 64, inclusive, will be discontinued from study drug at Week 64, and so on).
2 - Achieving subclinical remission (biomarkers)
Biomarkers serve as objective indicators of disease activity and treatment response, providing insights into inflammatory processes that may persist beyond visible symptom resolution.1 New biomarkers can be linked to ‘disease modification’ concepts, highlighting the importance of targeting the underlying processes of disease to alter their course. Subclinical remission involves the normalization of these fundamental inflammatory mechanisms, even in the absence of visible symptoms.10
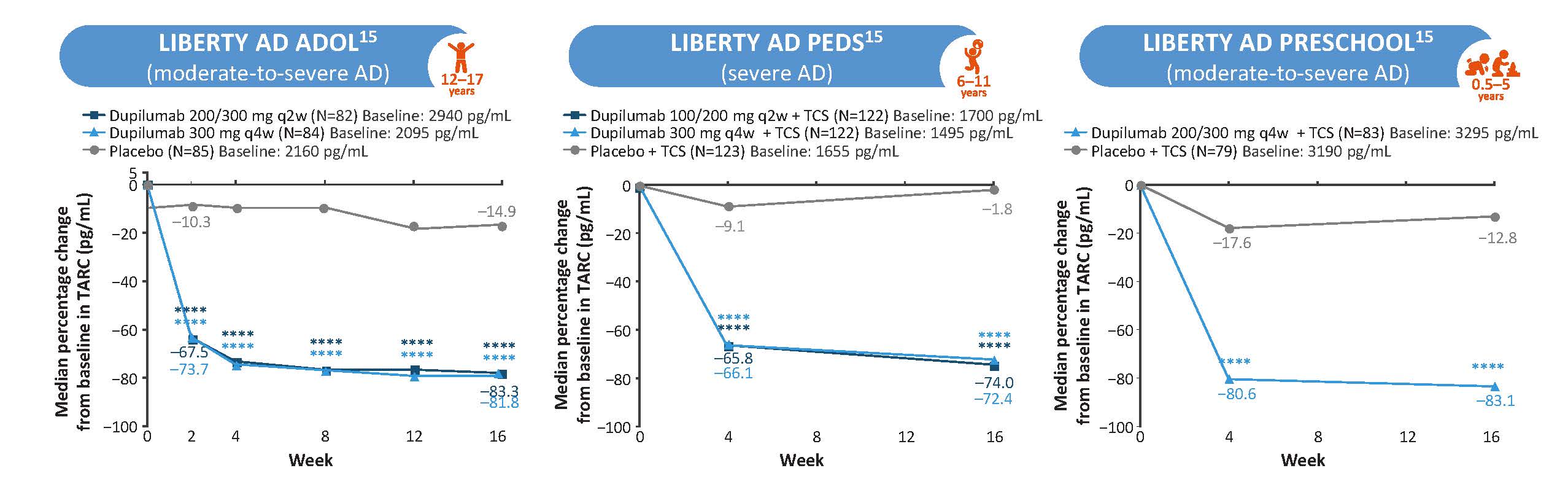
CCL17 (TARC)
.jpg)
|
|
Figure 4: Dupilumab Reduced Levels of CCL17 Across Age Groups (LIBERTY AD ADOL, PEDS, and PRESCHOOL)a,15
****P<0.0001. aPost-hoc analyses. AD, atopic dermatitis; TARC, thymus and activation-regulated chemokine.
The figure is drawn up by Sanofi based on reference 15, figure 1
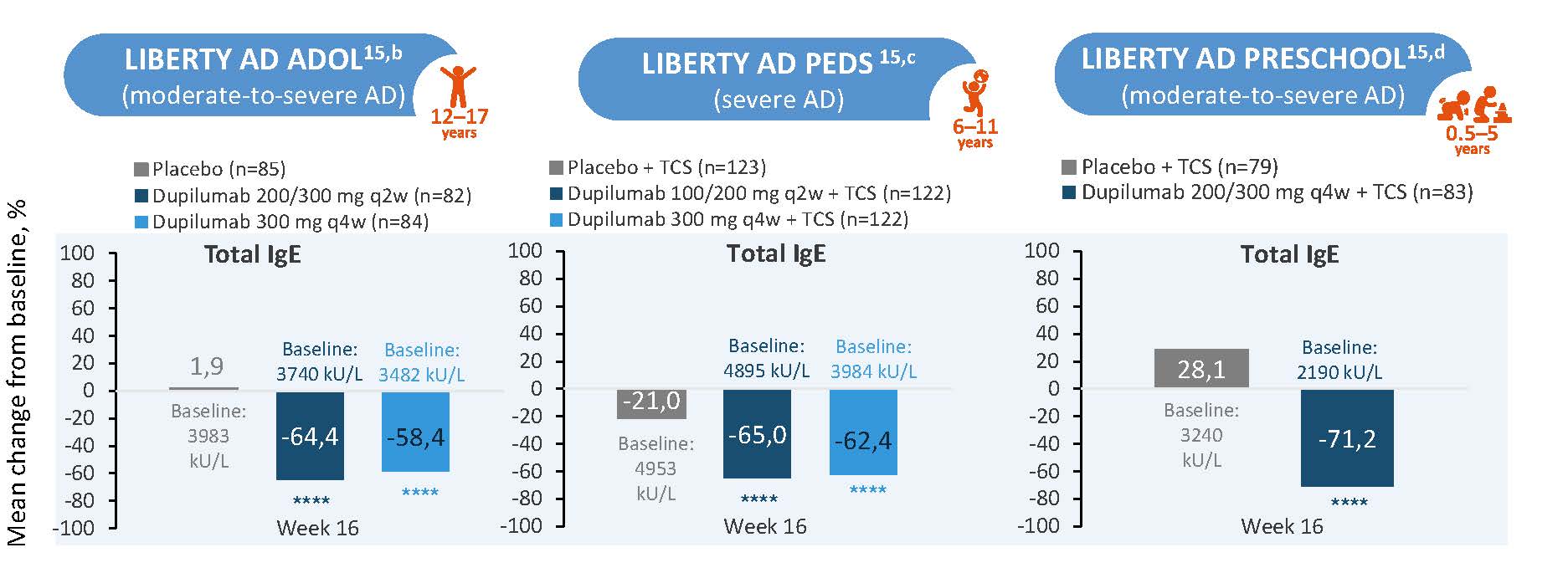
IgE

|
|
Figure 5: Dupilumab Reduced Levels of IgEa in Patients Aged 6 months-17 years (LIBERTY AD ADOL/PEDS)a,15
****P<0.0001. aPost-hoc analysis. bAge 12 to 17 years cAge 6 to 11 Years d0 to 5 years. AD, atopic dermatitis; q2w, every 2 weeks; q4w, every 4 weeks; qw, weekly; TCS, topical corticosteroid.
The figure is drawn up by Sanofi based on reference 15, figure 2
3 - Reversing the underlying pathophysiology
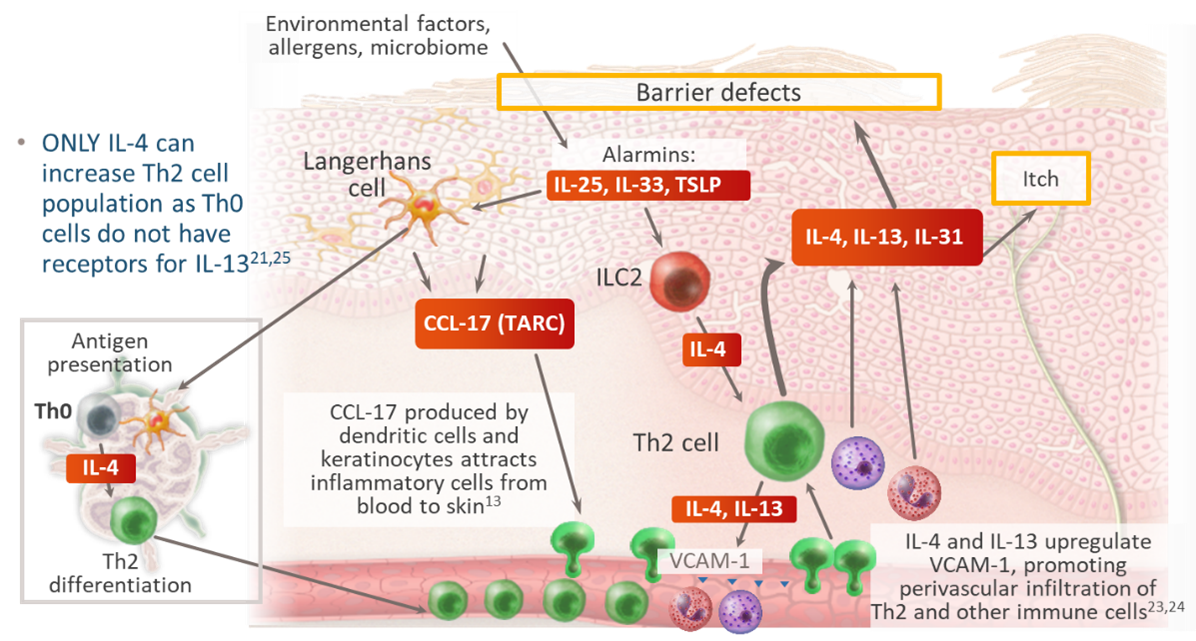
Type 2 inflammation is a key pathomechanism in AD which leads to skin barrier dysfunction, dysbiosis, and neuroimmune dysregulation.20 Any intervention that endurably impacts these pathomechanisms could be considered disease modifying.1
In atopic dermatitis, there is a dynamic crosstalk between type 2 inflammation and epithelium dysfunction13,20-25 (Figure 6):
- Type 2 inflammation impacts expression of genes involved in barrier function, including intracellular proteins, extracellular lipids, and junctional proteins20
- IL-4 and IL-13 cause barrier dysfunction directly and potentiate itch sensation that leads to scratching21
- IL-4 causes endothelial cell apoptosis inducing leakage of plasma proteins into the skin22
- IL-4 and IL-13 upregulate VCAM-1 promoting skin homing of inflammatory cells23,24
Figure 6: Crosstalk Between Type 2 Inflammation and Epithelium Dysfunction in AD13,20-25
IL, interleukin; ILC, innate lymphoid cell; Th, T helper cell; TSLP, thymic stromal lymphopoietin; VCAM, vascular cell adhesion molecule.
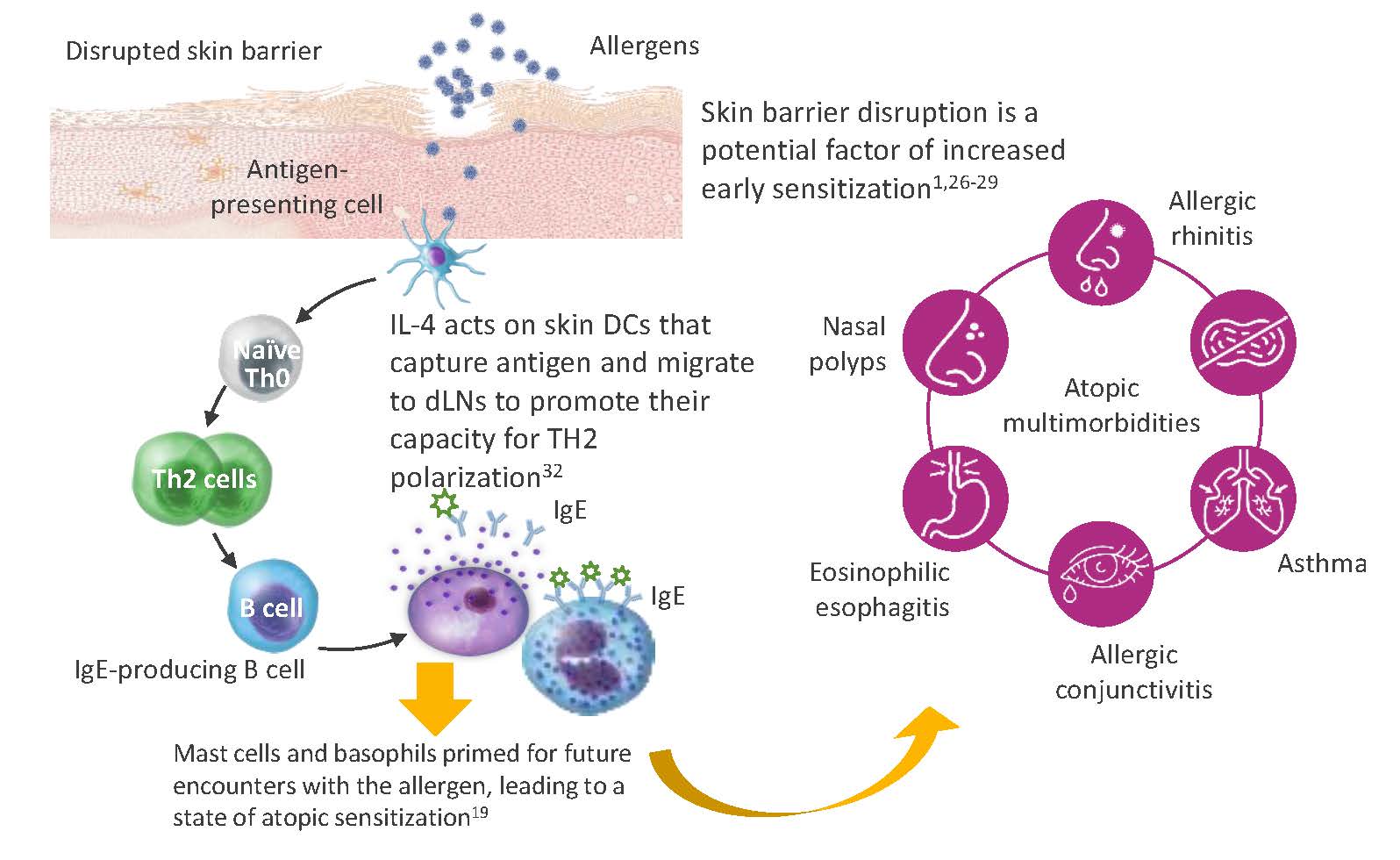
Furthermore, that AD often precedes atopic disease at other body sites has led to the hypothesis that the skin may be an important site for sensitization to allergens.26–28 Skin disruption has been shown to increase allergen exposure and skin sensitization, which may increase the risk of asthma and other atopic diseases (Figure 7).1,28,29 Repairing the skin barrier may thus prevent the development or worsening of other systemic atopic diseases.1,18,21,26-31
Figure 7: The role of disrupted skin barrier in allergen sensitization and atopic comorbidities1,18,19,21,26-32
Ig, immunoglobulin; IL, interleukin; Th, T-helper.
4 - Preventing the development of comorbidities
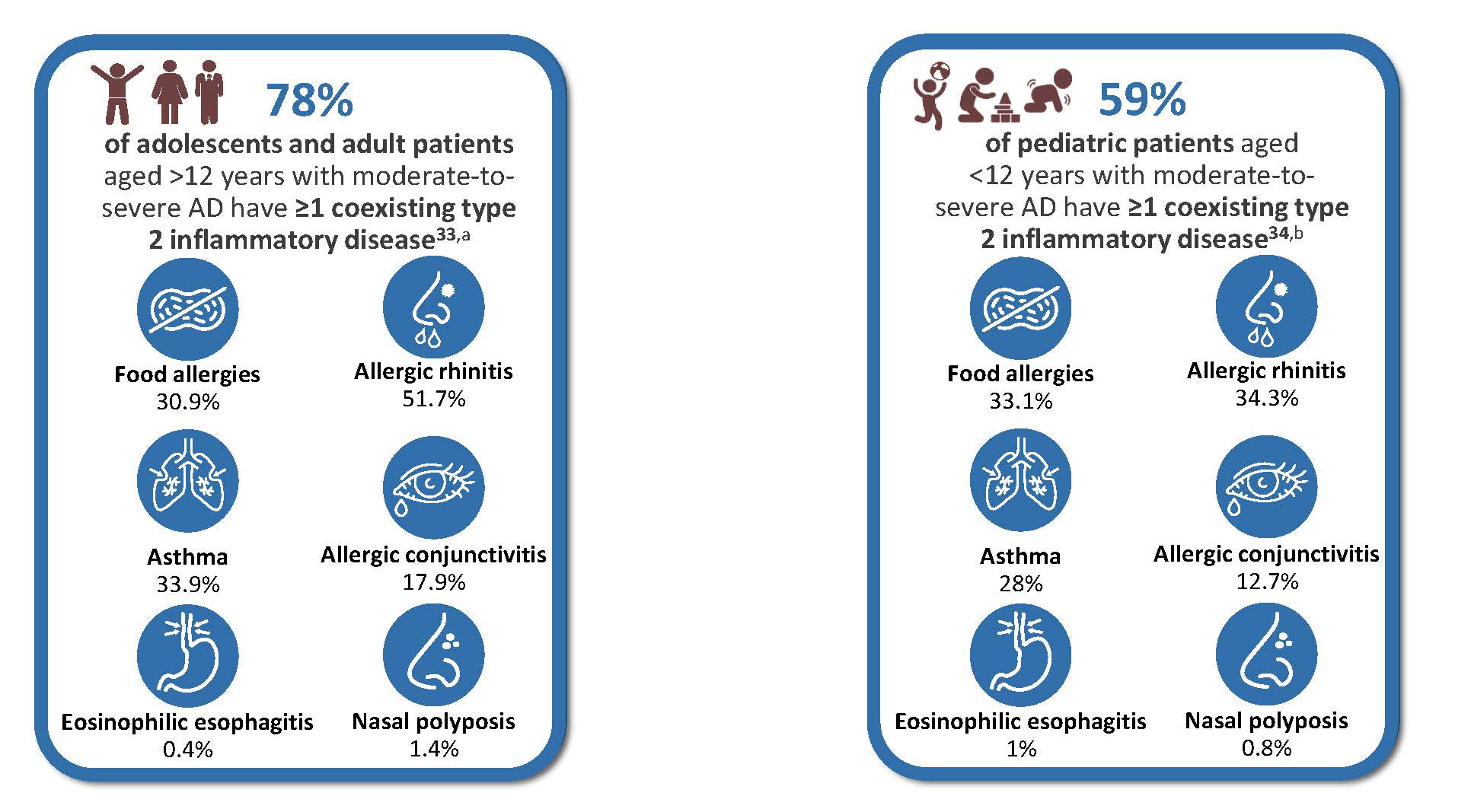
Type 2 inflammation is a shared mechanism across multiple atopic conditions. Most patients with moderate-to-severe AD have associated type 2 comorbidities. 78% of adults and adolescents (741/952), and 59% of pediatric patients under 12 years (432/732) with moderate-to-severe AD present at least one coexisting type 2 inflammatory disease, such as asthma, allergic rhinitis, or food allergy (Figure 8).33,34
Figure 8: Coexisting type 2 inflammatory disease in adults and adolescents patients with moderate-to-severe AD (left) and pediatric patients aged<12 years with moderate-to-severe AD (right)33,34
aGLOBOSTAD bPEDISTAD AD, atopic dermatitis; sIgE, specific immunoglobulin E.
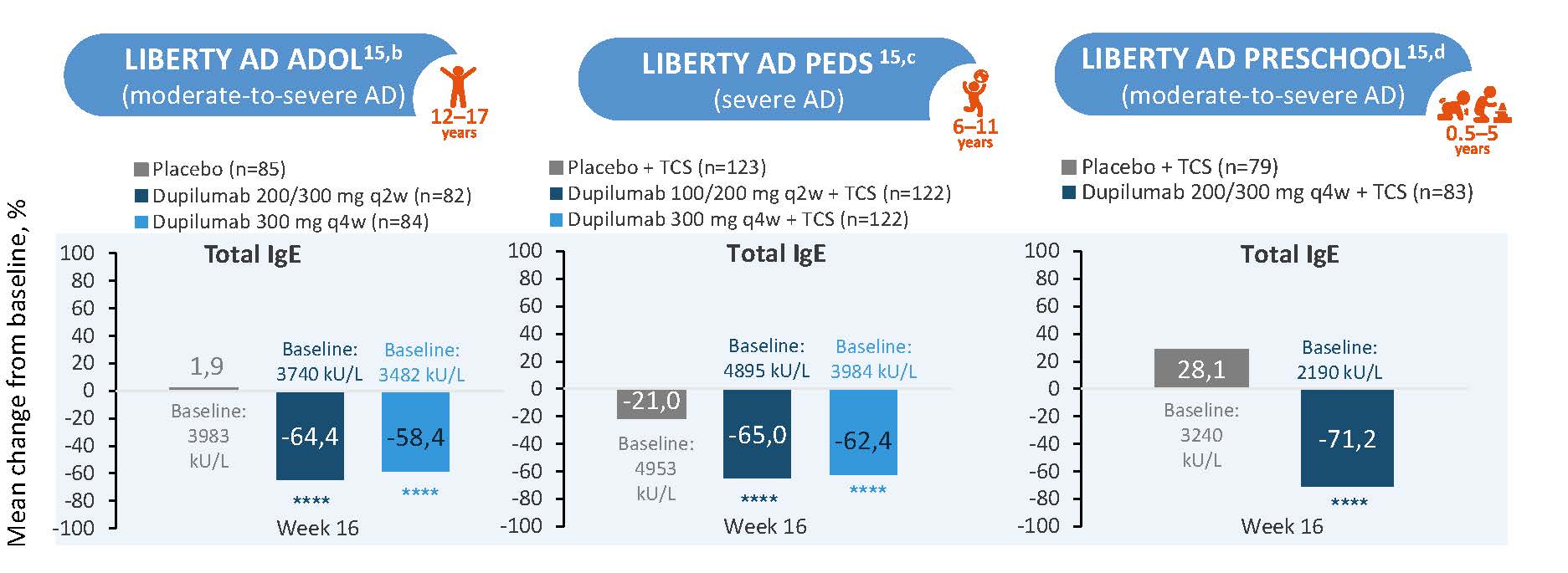
Normalizing total IgE, as well as allergen-specific IgE, could represent a meaningful approach to evaluate the status of a potential atopic march.1 Dupilumab treatment demonstrated consistent reductions in total IgE levels across pediatric age groups (6 months-17 years) with (moderate-to-) severe atopic dermatitis, with the most notable change observed in the preschool group (0.5-5 years) at Week 16, showing a -71.2% median change from baseline compared to -28.1% in the placebo group.15 (Figure 9)
Figure 9: Dupilumab Reduced Levels of IgEa in Patients Aged 6 months-17 years15
****P<0.0001. aPost-hoc analysis. bAge 12 to 17 years cAge 6 to 11 Years d0 to 5 years. AD, atopic dermatitis; Ig, immunoglobulin; q2w, every 2 weeks; q4w, every 4 weeks; qw, weekly; TCS, topical corticosteroid
The figure is drawn up by Sanofi based on reference 15, figure 2
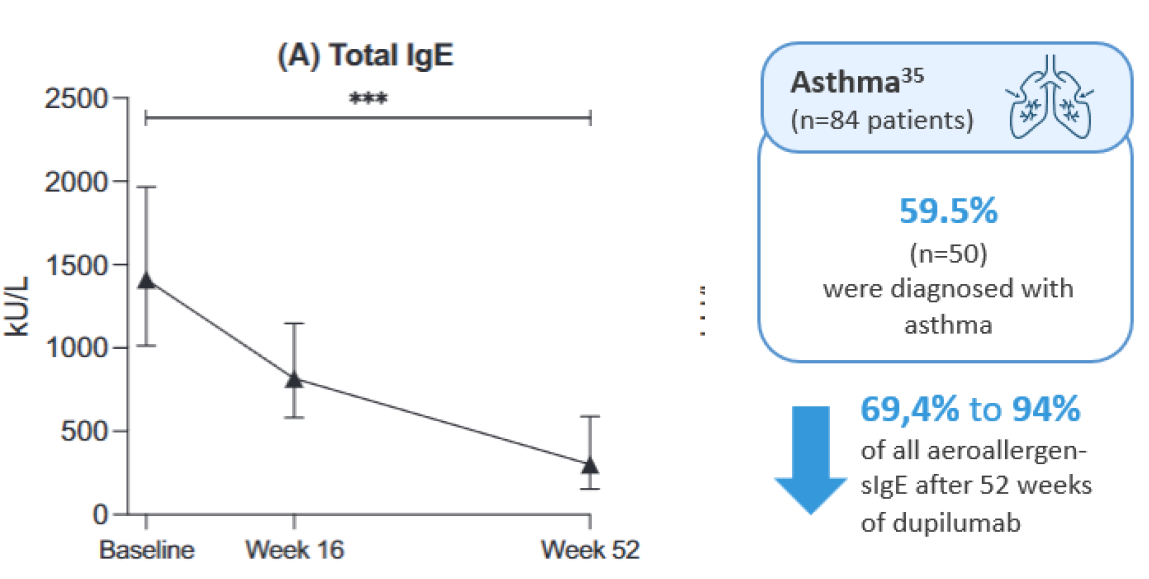
Supporting this, a recent publication from the BioDay registry demonstrated that one year of dupilumab treatment in atopic dermatitis pediatric patients (<18 years), resulted in a significant improvement in comorbid asthma and a profound decrease in aeroallergen-specific IgE levels.35
Figure 10: The figure is reproduced by Sanofi based on reference 35, figure 3.
***p < .001. Estimated median totaland specific IgE levels during 1 year of dupilumab treatment in pediatric atopic dermatitis patients with comorbid asthma and/or allergic rhinitis. Estimated median (s) IgE levels from covariancepattern models in kU/L during dupilumab treatment for (A) Total IgE
-
Bieber T. Nat Rev Drug Discov. 2023;22:662–680
-
Pariser DM, et al. Curr Med Res Opin. 2020;36:367–376
-
Wu JJ, et al. Dermatol Ther (Heidelb). 2021;11:327–330
-
Merola JF, et al. J Am Acad Dermatol. 2021;84:495–497.
-
Beck LA, et al. JAMA Dermatol. 2024:e241536
-
Strober B, et al. JAMA Dermatol. 2022;158:142–150
-
Kimball et al. Dermatol Ther (Heidelb) (2023) 13:2107–2120
-
Blauvelt A, et al. Am J Clin Dermatol. 2022;23:365–383.
-
Cork MJ, et al. Br J Dermatol. 2021;184(5):857-870
-
Park CO, et al. EBioMedicine. 2024;103:105121
-
Mastraftsi S, et al. J Clin Med. 2022;11:4639
-
Halling AS, et al. J Allergy Clin Immunol. 2023;151:1550–1557
-
Guro H, et al. Front Immunol. 2025;16:1489277.
-
Yoko Kataoka, Journal of Dermatology 2014; 41: 221–229
-
Beck LA, et al. J Allergy Clin Immunol. 2025;155(1):135-143.
-
Wang F, et al. Cell. 2021;184:422–440.
-
Atwal S, et al. Pediatr Dermatol. 2023;40(2):301-304
-
Furue K, et al. Immunology. 2019;158(4):281-286.
-
Sweeney A, et al. Allergy Asthma Clin Immunol. 2021;17(1):30
-
Beck LA, et al. JID Innov. 2022;2:100131.
-
Haddad EB, et al. DermatolTher (Heidelb). 2022;12:1501-1533.
-
Skaria T, et al. PLoS One. 2016;11(5):e0156002.
-
Kotowicz K, et al. Int Immunol. 1996;8(12):1915-1925.
-
Goldman M, et al. Trends Immunol. 2001;22(5):247-251.
-
Junttila IS. Front Immunol. 2018;9:888
-
Lack G, et al. N Engl J Med. 2003;348:977–985.
-
Ziegler SF. Ann Allergy Asthma Immunol. 2021;127:306–311.
-
Han H, et al. Immunol Rev. 2017;278:116–130
-
Ghezzi M, et al. Children (Basel). 2021;8:1165.
-
Paller AS, et al. J Allergy Clin Immunol. 2017;140:633–643.
-
Le Floc’h A, et al. Allergy. 2020;75:1188–1204.
-
Leyva-Castillo JM, et al. J Allergy Clin Immunol. 2024;154(6):1462-1471.e3.
-
Calzavara-Pinton P, et al. Adv Ther. 2023;40:5366–5382.
-
Paller AS, et al. J Am Acad Dermatol. 2022;87(5):1104-1108.
-
Rijst LPvd, et al. Pediatr Allergy Immunol. 2024; 35:e14178