EVEREST study highlights
First ever direct Head-to-Head Comparison of Dupilumab vs Omalizumab in Patients with Severe CRSwNP and Coexisting Asthma.
We are pleased to share that the phase IV EVEREST study primary manuscript (De Corso et al. 2025) has been published in Lancet Respiratory Medicine on September 28, 2025.
EVEREST is the first-ever direct head-to-head trial of respiratory biologics. This international Phase 4, head-to-head, randomized, double-blind study evaluated the efficacy and safety of dupilumab versus omalizumab in adult patients with severe uncontrolled chronic rhinosinusitis with nasal polyps (CRSwNP) and coexisting asthma.
In the study, dupilumab demonstrated superiority over omalizumab in all primary and secondary efficacy endpoints, and treatment safety profiles were comparable.1 Please find the summarized results below or explore the full publication.
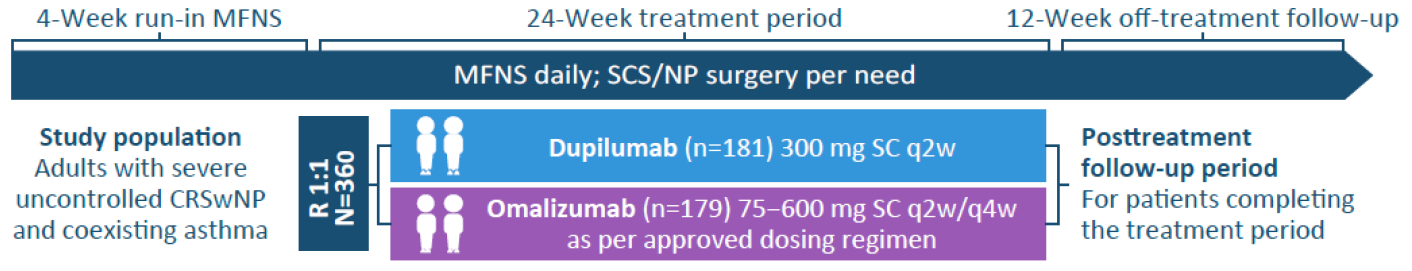
Study design1,2
In EVEREST, 360 adults with severe, uncontrolled CRSwNP and coexisting asthma were randomized to receive dupilumab 300 mg (n=181) every two weeks or a weight- and immunoglobulin E (IgE) level-based dosing regimen of omalizumab (n=179) every two or every four weeks. Both dupilumab and omalizumab were added to background mometasone furoate nasal spray (MFNS).
Study results1
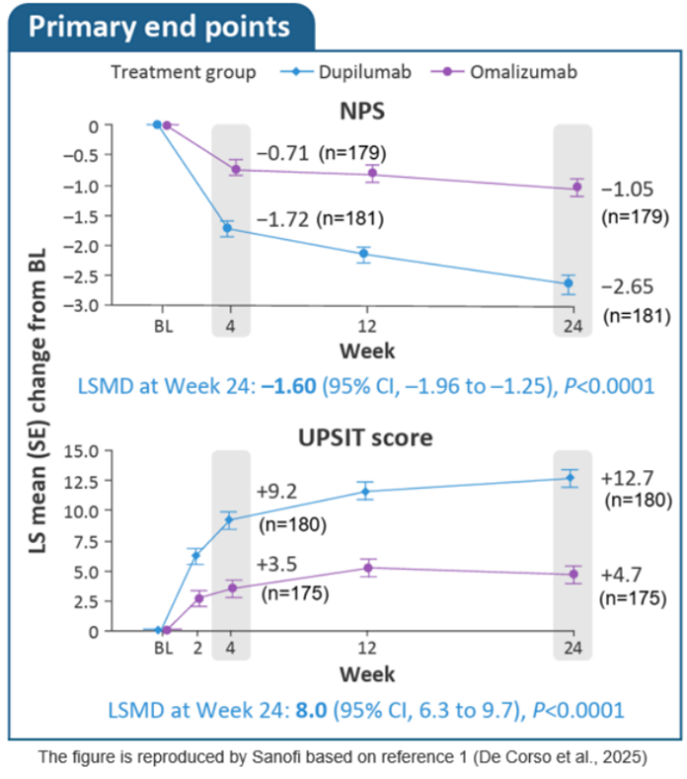
Primary endpoints |
Nasal polyp score (NPS) reduction was significantly greater with dupilumab vs. omalizumab at W24 (Least Square Mean difference ‒1.60; 95% CI: – 1.96, –1.25; P < 0.0001).
Improvement in sense of smell (UPSIT) was significantly greater with dupilumab vs. omalizumab at W24 (Least Square Mean difference 8.0 (95% CI 6.3, 9.7; P < 0.0001).
Other CRS endpoints |
Significantly greater improvements e.g. in nasal congestion, loss of smell (LoS) & total symptom score, nominally greater e.g. in SNOT-22 at W24.
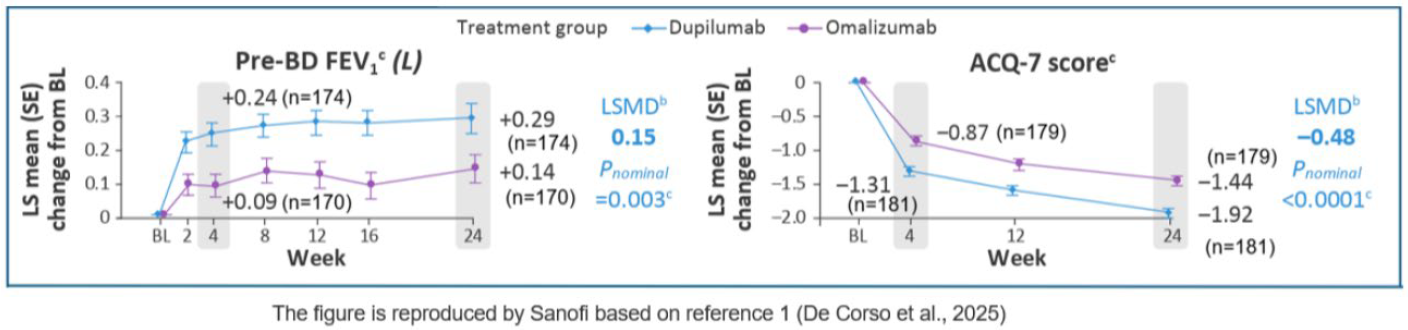
Asthma-related endpoints |
Improvements in lung function (pre-BD FEV1) and asthma control (ACQ-7 score) were evident as early as W4 with dupilumab vs omalizumab, with nominally greater improvements at Week 24.
Safety
Safety profiles of dupilumab and omalizumab were comparable and consistent with known profiles. Treatment-emergent adverse events (TEAEs) were similar in the dupilumab (n= 179) and omalizumab (n=173) groups (64% vs 67%). Most common adverse events for dupilumab and omalizumab, respectively, were nasopharyngitis (12% vs 12%), accidental overdose (7% vs 12%), headache (6% vs 8%), upper respiratory tract infection (6% vs 5%), and cough (5% vs 1%). Serious TEAEs occurred in three dupilumab patients (2%) and seven omalizumab patients (4%). Treatment discontinuation due to TEAEs were reported in five dupilumab participants (3%) and two omalizumab participants (1%).
-
De Corso E, et al. Lancet Respiratory Medicine, 2025.
-
De Prado Gomez L, et al. Am J Rhinol Allergy, 2022.