EFLUELDA - Seasonal high dose flu vaccine
Efluelda - Seasonal high dose flu vaccine
Trivalent influenza vaccine (inactivated, split virus) 60 micrograms HA/strain.
Efluelda is indicated for active immunization of adults aged 60 years and older for the prevention of influenza disease. The use of Efluelda should be based on official recommendations regarding influenza vaccination.
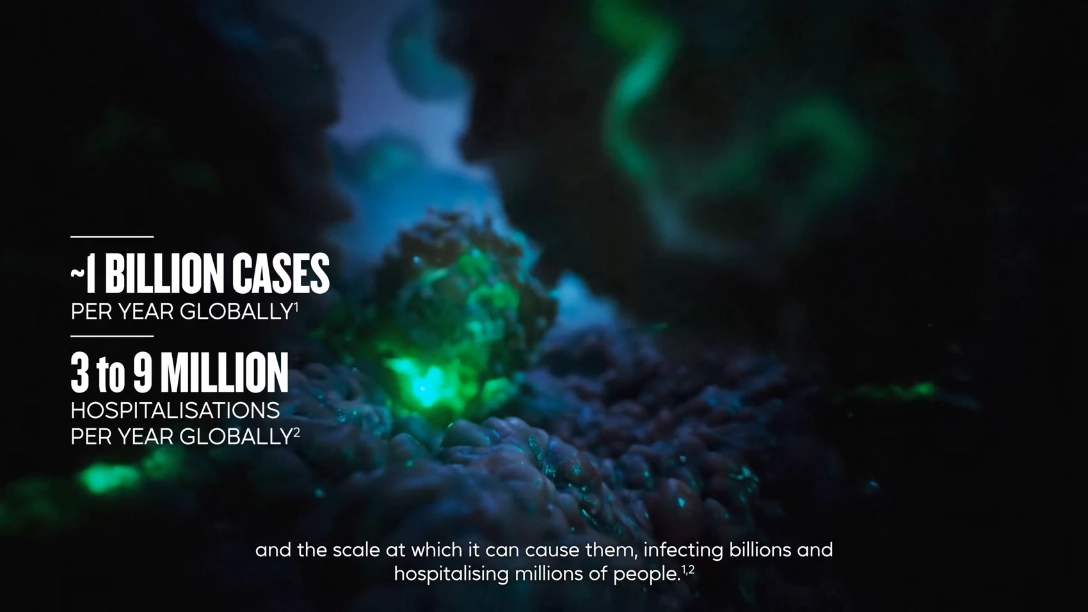
Efluelda is a High-Dose Flu vaccine With Better Protection Than Standard Dose Against Flu and Flu-Related Hospitalisations.1-3
Scroll down to the bottom of the page to find the efluelda compulsory information.
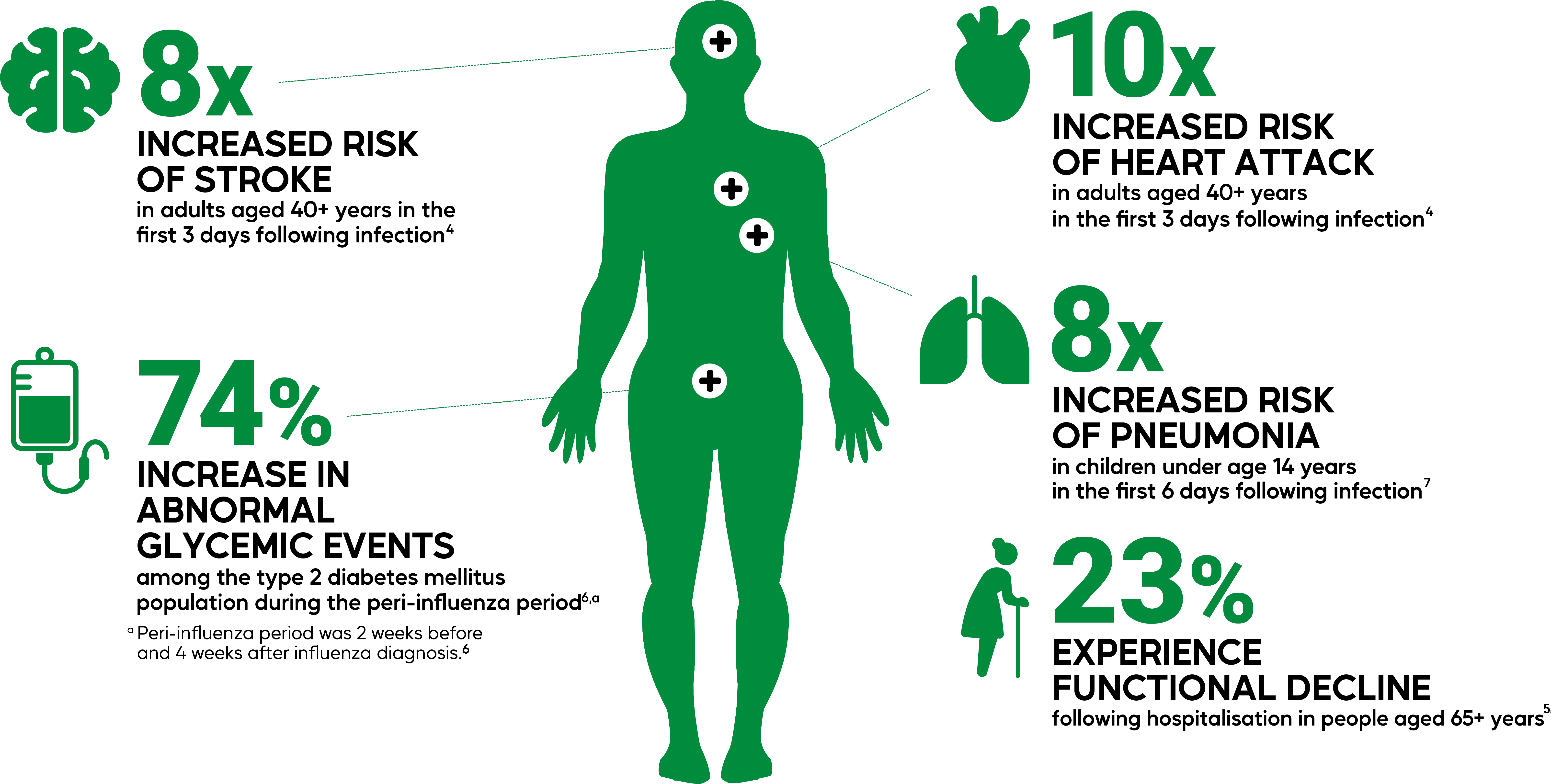
Sanofi’s high-dose flu vaccine can help prevent flu-related hospitalisations in older adults1-3
Influenza can affect major organ systems4-6
An unpredictable virus with a significant impact on patients’ lives4-6
Our standard is protection beyond flu
Our commitment is to ensure patients have access to influenza vaccines that protect them from what really matters: hospitalisations due to flu infection or attributable to flu
Protection Beyond Flu is our standard of quality evidence, supported by data demonstrating reduction of hospitalisations vs standard-dose (SD) flu vaccine in older adults.1-3

Sanofi high-dose (HD) flu vaccine has demonstrated BETTER PROTECTION against influenza infection compared with Standard Dose flu vaccines1,2 |

HD flu vaccine has been shown to reduce flu-related complications and hospitalisations across 11 FLU SEASONS of observational studies consisting of >45 MILLION ADULTS AGED 65 YEARS AND OLDER1,3 |
Förstärkta influensavacciner rekommenderas till personer som bor på särskilda boenden för äldre (SÄBO). Vilken typ av förstärkt vaccin som används varierar från år till år, beroende på vilket vaccin som upphandlats för den aktuella säsongen.10
Efluelda is well tolerated1,8
The most frequently reported adverse reaction after vaccination was

42,6%injection-site pain1 |

23,8%myalgia1 |

17,3%headache1 |
15,6%malaise1 |
Most of these reactions occurred and resolved within 3 days of vaccination. The intensity of most of these reactions was mild to moderate.1
Overall, adverse reactions were generally less frequent in participants aged ≥65 years than in participants aged 60 to 64 years.1
The reactogenicity of the HD vaccine was slightly increased compared to the standard-dose vaccine, but no major difference in intensity was observed.1
IN ADULTS 60+
High-dose influenza vaccine demonstrated a superior immune response vs standard-dose vaccine in all virus strains1

|
HD-QIV induced an immune response that was superior to the responses induced by SD-QIV for all 4 virus strains 28 days post-vaccination in adults both 60 to 64 years of age and 65 years of age and older.1 |

|
The efficacy and effectiveness data from adults 65 years of age and above can thus be inferred to adults from 60 years of age and above.1 |
This was a randomised, active-controlled, modified double-blind, phase 3 clinical trial conducted in Europe in adults 60 years of age and older to demonstrate the superiority of HD-QIV over SD-QIV for all strains, as assessed by HAI (haemagglutinin inhibition) GMTs at Day 28 in adults 60 to 64 years of age and in adults 65 years of age and older.1
A total of 1539 adults (760 adults 60 to 64 years of age and 779 adults 65 years of age and older) were randomised to receive either 1 dose of HD-QIV or 1 dose of SD-QIV.1
IN ADULTS 65+
Efluelda is the high-dose influenza vaccine proven to provide superior flu protection compared to a standard dose flu vaccine1-3
In a head-to-head trial2 in adults 65 years and older, Efluelda (HD-TIV) demonstrated proven superior protection vs a standard-dose influenza vaccine.1
PRIMARY ENDPOINT: Relative vaccine efficacy (rVE) against flu due to ANY lab-confirmed circulating strains1,2

|
SUPERIOR PROTECTION AGAINST INFLUENZA INFECTION VS STANDARD DOSE1,2* |
HD-TIV RESULTSIn an Randomized Clinical Trail of 31,803 subjects, HD-TIV showed 24.2% (95% CI: 9.7, 36.5) superior protection against lab-confirmed influenza cases of any circulating strain vs standard-dose influenza vaccine1,2 |
FOR OVER 11 SEASONS AND IN >45 MILLION OLDER ADULTS,
High-dose influenza vaccine has been shown to better prevent flu-related hospitalisations than standard-dose vaccine in people aged 65 years and older, even in mismatched years1,3
A meta-analysis of published randomised and observational studies conducted over multiple influenza seasons assessed the relative vaccine effectiveness of high-dose and standarddose influenza vaccines against influenza-associated outcomes.3
POOLED RELATIVE VACCINE EFFECTIVENESS—HIGH DOSE VERSUS STANDARD DOSE: Over 11 seasons, several retrospective studies in 45+ million patients aged 65+ showed reductions in hospitalisations compared to standard-dose flu vaccines:

FEWER |

FEWER |
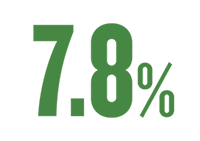
ALL-CAUSE HOSPITALISATION (95 % KI: 5,3 % till 10,3 %, p < 0,001)1** |
IN A 2024 META-ANALYSIS OF RANDOMISED TRIALS OF HIGH-DOSE VS STANDARD-DOSE INFLUENZA VACCINE,
High-dose influenza vaccine reduced the incidence of pneumonia and influenza and of all-cause hospitalisation in adults ≥65 years vs standard-dose vaccine9
In a meta-analysis, investigators estimated the pooled relative vaccine efficacy (rVE) of high-dose influenza vaccine (HD-IV) vs standard-dose influenza vaccine (SD-IV) in reducing the rates of pneumonia and influenza hospitalisation, all-cause hospitalisations, and all-cause death in adults ≥65 years.9
To receive more updates on Efluelda and other Sanofi products, log-in or register and subscribe to our communication!
Efluelda - Trivalent högdosinfluensavaccin
Efluelda®. Rx, EF, J07BB02.
▼ Detta läkemedel är föremål för utökad övervakning.
Trivalent influensavaccin (spjälkat virus, inaktiverat) injektionsvätska, suspension i förfylld spruta, 60 mikrogram HA/stam. Indikation: för aktiv immunisering av vuxna från 60 års ålder för att förebygga influensa. Efluelda ska användas i enlighet med officiella rekommendationer om vaccination mot influensa. Varningsföreskrifter och begränsningar: Efluelda får under inga omständigheter administreras intravaskulärt. Vaccination ska skjutas upp hos patienter med akut febersjukdom tills febern har försvunnit. För fullständig förskrivarinformation se www.fass.se. Kontaktuppgifter: Efluelda tillhandahålls av Sanofi AB. Box 300 52, 10425 Stockholm, tel +46 8 634 50 00. Vid frågor om våra läkemedel kontakta: infoavd@sanofi.com. Datum för översyn av produktresumén: 2025-07-10.
* Högdos (HD) influensavaccin reducerade antalet influensafall med 24,2 % (CI 9,7; 36,5), jämfört standarddos (SD) influensavaccin (227 respektive 300 fall för HD och SD) i en dubbel-blind, aktiv-kontrollerad multicenterstudie där 31 989 personer 60+ randomiserades 1:1 att erhålla trevalent HD (TIV-HD) eller SD influensavaccin. Studien utfördes under två influensasäsonger, 2011-2013. I studien specificerat kriterie för “superior effekt”: påvisad högre effekt av HD än SD med den lägre gränsen av 2-sidigt 95% konfidenintervall (CI): >9.1%
** Flera retrospektiva studier som omfattade 8 influensasäsonger och fler än 24 miljoner personer från 65 års ålder
Referenser
Efluelda produktresume, www.fass.se, october 2025
DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635-645. doi:10.1056/NEJMoa1315727
Lee JKH, Lam GKL, Yin JK, Loiacono MM, Samson SI. High-dose influenza vaccine in older adults by age and seasonal characteristics: systematic review and meta-analysis update. Vaccine X. 2023;14:100327. doi:10.1016/j.jvacx.2023.100327
Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018;51(3):1701794. doi:10.1183/13993003.01794-2017
Andrew MK, MacDonald S, Godin J, et al. Persistent functional decline following hospitalization with influenza or acute respiratory illness. J Am Geriatr Soc. 2021;69(3):696-703. doi:10.1111/jgs.16950
Samson SI, Konty K, Lee WN, et al. Quantifying the impact of influenza among persons with type 2 diabetes mellitus: a new approach to determine medical and physical activity impact. J Diabetes Sci Technol. 2021;15(1):44-52. doi:10.1177/1932296819883340
Kubale J, Kuan G, Gresh L, et al. Individual-level association of influenza infection with subsequent pneumonia: a case-control a nd prospective cohort study. Clin Infect Dis. 2021;73(11):e4288-e4295. doi:10.1093/cid/ciaa1053
Chang LJ, Meng Y, Janosczyk H, Landolfi V, Talbot HK; for the QHD00013 Study Group. Safety and immunogenicity of high-dose quadrivalent influenza vaccine in adults ≥65 years of age: a phase 3 randomized clinical trial. Vaccine. 2019;37(39):5825-5834. doi:10.1016/j.vaccine.2019.08.016
Skaarup KG, Lasses MCH, Modin D, et al. The relative vaccine effectiveness of high-dose vs standard-dose influenza vaccines in preventing hospitalization and mortality: a meta-analysis of evidence from randomized trials. J Infect 2024;89(1):106187. doi:10.1016/j.jinf.2024.106187
- Rekommendationer om influensavaccination till riskgrupper — Folkhälsomyndigheten. https://www.folkhalsomyndigheten.se/publikationer-och-material/publikationsarkiv/r/rekommendationer-om-influensavaccination-till-riskgrupper/, october 2025