Treatment with Nexviadyme®
Clinical trial
Nexviadyme® (avalglucosidase alfa) is an enzyme replacement monotherapy (ERT) indicated for the long-term treatment of patients with Pompe disease (acid α-glucosidase deficiency), both infantile form (IOPD) and late form (LOPD).1
COMET - The first pivotal, head-to-head, ERT clinical trial in LOPD2
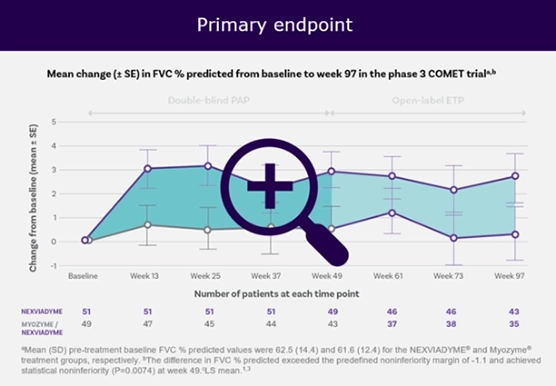
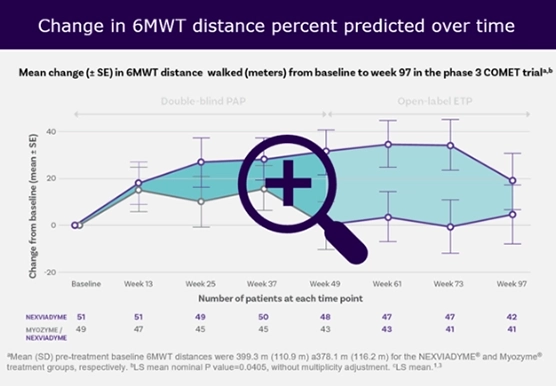
COMET study: Nexviadyme® (avalglucosidase alfa) helped patients improve their ability to breathe and walk compared with baseline.
Watch this short video and have a brief overview of the design and results of the study
Efficacy and Safety of Avalglucosidase Alfa in Patients With Late-Onset Pompe Disease After 97 Weeks A Phase 3 Randomized Clinical Trial3
Click the link below and get access to the COMET 97 week article for more information.
NEO-EXT study: Long term safety and efficacy evaluation of patients with LOPD - follow up 6,5y4
Watch this short video and have a brief overview of the design and results of the study.
Summary of Product Characteristics - Nexviadyme® (avalglucosidase alfa)
Click the link below and get access to the Summary of Product Characteristics for more information on Nexviadyme®.
Summary of Product Characteristics - Myozyme® (alglucosidase alfa)
Click the link below and get access to the Summary of Product Characteristics for more information on Myozyme®.
References:
-
Nexviadyme Summary of Product Characteristics. April 2024
-
Diaz-Manera J, Kishnani PS, Kushlaf H et al. Safety and efficacy of avalglucosidase alfa versus alglucosidase alfa in patients with late-onse Pompe disease (COMET): a phase 3, randomized, multicenter trial. Lancet Neurol 2021; 20; 1012-26.
-
Kishnani PS, Diaz-Manera J, Toscano A et al. Efficacy and Safety of Avalglucosidase Alfa in Patients With Late-Onset Pompe Disease After 97 Weeks A Phase 3 Randomized Clinical Trial. JAMA Neurol. 2023;80(6):558-567. doi:10.1001/jamaneurol.2023.0552
-
Dimachkie M, Barohn R, Byrne B et al. Long-term Safety and Efficacy of Avalglucosidase Alfa in Patients With Late-Onset Pompe Disease. Neurology 2022, May 26, DOI: 10.1212/WNL.0000000000200746